Moles and Formula Mass Atomic Mass Knowing the












- Slides: 23

Moles and Formula Mass

Atomic Mass • Knowing the mass of atoms is extremely helpful in chemistry • But…a single spec of dust contains around 1 x 1016 atoms! • How can we possibly figure out the mass of one single atom? ?

Atomic Mass • We determine the mass of one atom RELATIVE to another experimentally – Step 1: establish a mass of one atom to be used as the standard • Atomic mass-the mass of the atom in atomic mass units (amu) – Aka atomic weight – Indicates how heavy one atom of one element is compared to one atom of another element

Atomic Mass Unit (amu) • 12 amu = mass of one C-12 atom • Therefore, 1 amu = 1/12 the mass of a C-12 atom • The atomic mass of other elements has been determined experimentally by determining the ratio or percent mass in comparison to C-12 – Ex: a hydrogen atom is found to be 8. 400 percent as massive as a C-12 atom; therefore 12 x 8. 400% = 1. 008 amu

Atomic Mass Unit • We do not know the actual mass of an atom! • Example: even though we do not know the actual mass of an iron atom, we do know that an iron atom if approximately 56 times as massive as a hydrogen atom

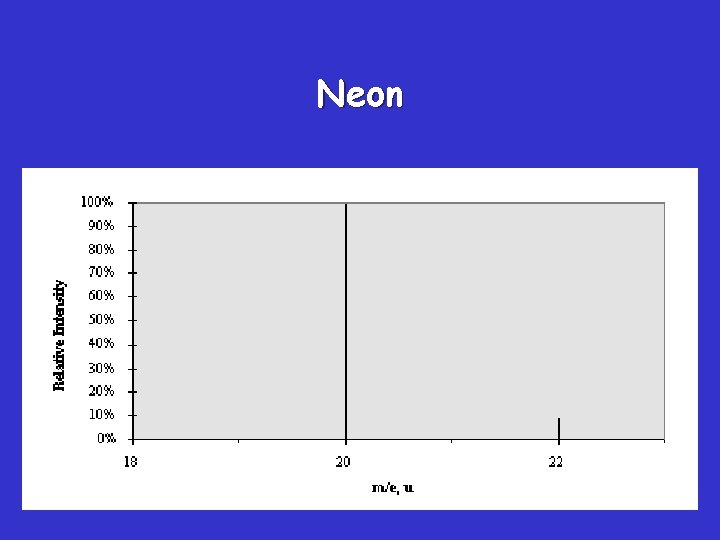
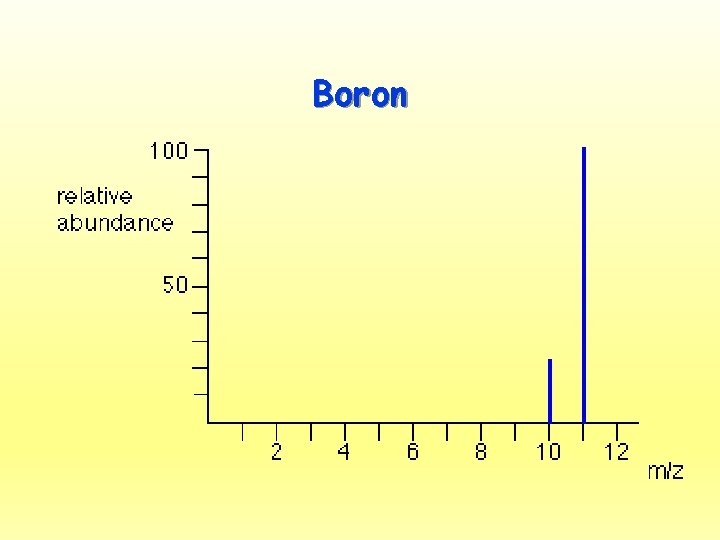
Average Atomic Mass • Naturally occurring elements occur in more than one isotope – Remember: what’s an isotope? What’s the same and what’s different? • Official atomic masses are averages of all the isotopes naturally occurring for a given element

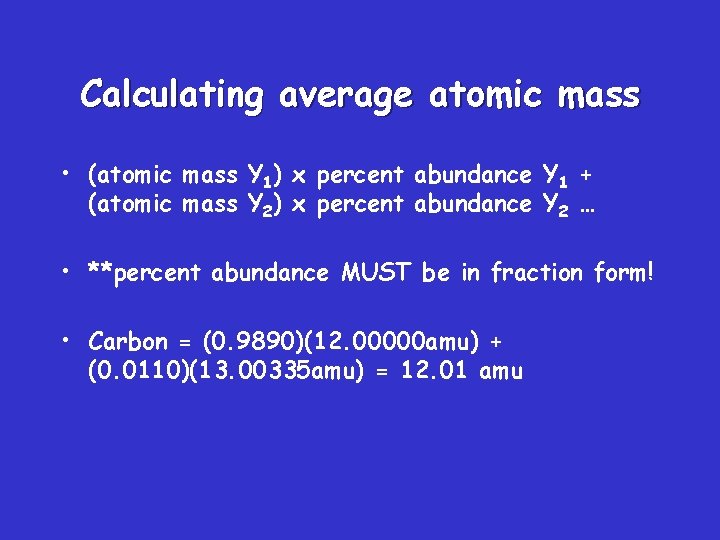
Calculating average atomic mass • (atomic mass Y 1) x percent abundance Y 1 + (atomic mass Y 2) x percent abundance Y 2 … • **percent abundance MUST be in fraction form! • Carbon = (0. 9890)(12. 00000 amu) + (0. 0110)(13. 00335 amu) = 12. 01 amu

Sample average atomic mass • Copper, a metal known since ancient times, is used in electrical cables and pennies, among other things. The atomic masses of its two stable isotopes, Cu-63 (69. 09%) and Cu-65 (30. 91), are 62. 93 amu and 64. 9278 amu respectively. Calculate the average atomic mass of copper. The relative abundances are given in parentheses.

Solution • (0. 6909)(62. 93 amu) + (0. 3091)(64. 9278 amu) = 63. 55 amu • Follow up questions: Explain the fact that the atomic masses of some of the elements like fluorine listed in the periodic table are not an average value like that for carbon.

Solution (continued) • Atomic mass is based on isotopes that occur naturally and therefor must be stable (nonradioactive). • Some elements only have one stable isotope • Ex: fluorine only has one stable isotope (F-19) • The second longest living isotope (F-18) only has a half-life of 109. 771 minutes. All other isotopes have half-lives under a minute, the majority under a second,

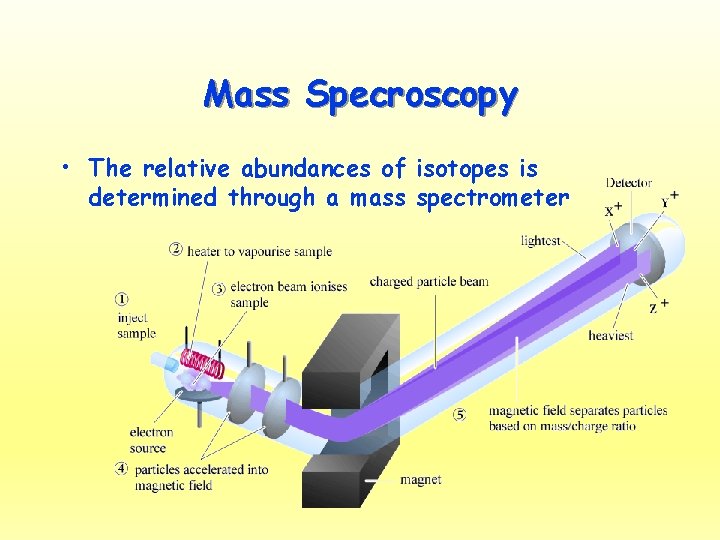
Mass Specroscopy • The relative abundances of isotopes is determined through a mass spectrometer

Mass Spec • 1. gaseous sample is bombarded by a stream of highenergy electrons • 2. This strips away the electrons leaving the positively charged nuclei • 3. Nuclei are accelerated as they travel through two oppositely charged plates • 4. A magnetic field is applied to the positively charged beam, which caused the particles to curve • 5. The angle at which a particle curves is based on the mass (more massive ions deflect/curve less) • 6. The amount of a particular ion/isotope is determined by the amount of voltage registered by the detector

Neon

Boron

Avogadro’s Number 6. 022 x 1023 is called “Avogadro’s Number” in honor of the Italian chemist Amadeo Avogadro (1776 -1855). I didn’t discover it. Its just named after me! Amadeo Avogadro

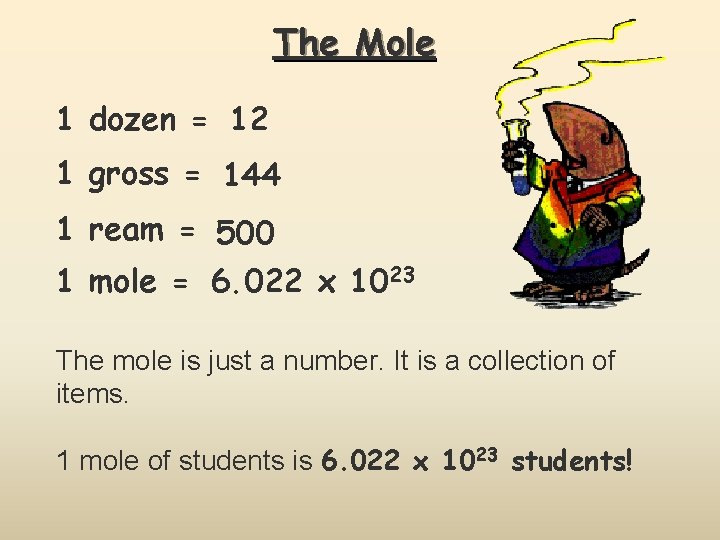
The Mole 1 dozen = 12 1 gross = 144 1 ream = 500 1 mole = 6. 022 x 1023 The mole is just a number. It is a collection of items. 1 mole of students is 6. 022 x 1023 students!

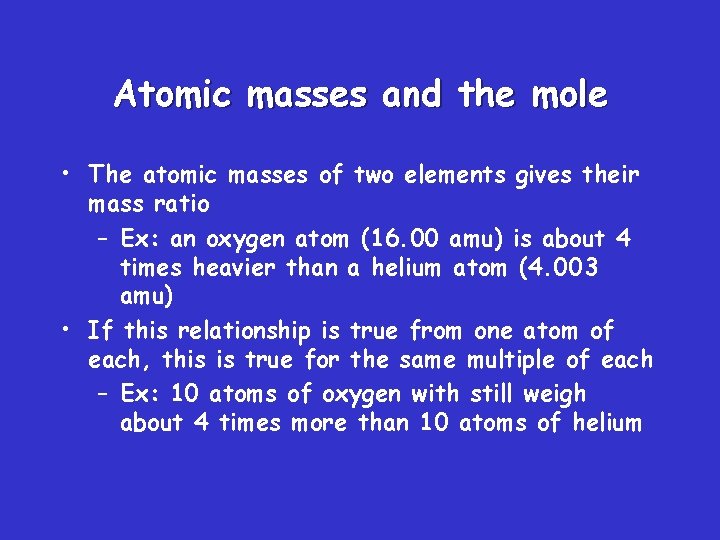
Atomic masses and the mole • The atomic masses of two elements gives their mass ratio – Ex: an oxygen atom (16. 00 amu) is about 4 times heavier than a helium atom (4. 003 amu) • If this relationship is true from one atom of each, this is true for the same multiple of each – Ex: 10 atoms of oxygen with still weigh about 4 times more than 10 atoms of helium

• Therefore… Continued… • If the masses (in grams) of two samples have this proportion, they must contain the same number of atoms! • Based on this logic, any mass (in grams) of an element that is equal to its atomic mass (in amu) with have the same number of atoms as another element with its atomic mass in grams • This number of atoms is Avogadro’s Number! • Conversion factor atomic mass in grams = 6. 022 x 1023

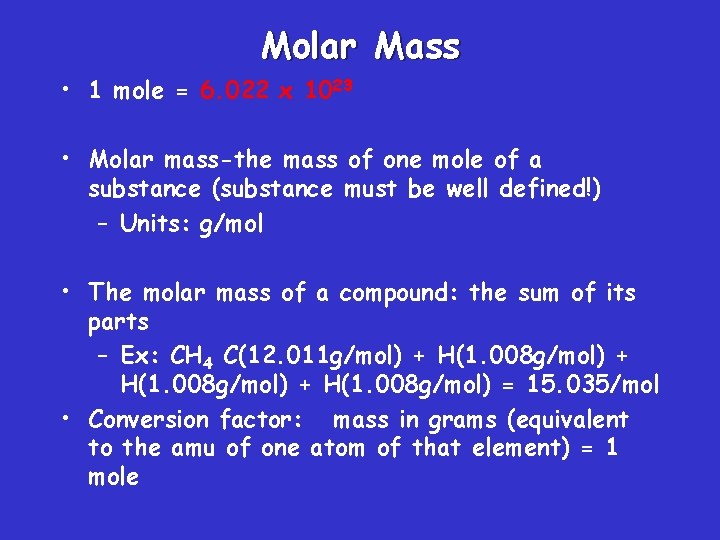
Molar Mass • 1 mole = 6. 022 x 1023 • Molar mass-the mass of one mole of a substance (substance must be well defined!) – Units: g/mol • The molar mass of a compound: the sum of its parts – Ex: CH 4 C(12. 011 g/mol) + H(1. 008 g/mol) = 15. 035/mol • Conversion factor: mass in grams (equivalent to the amu of one atom of that element) = 1 mole

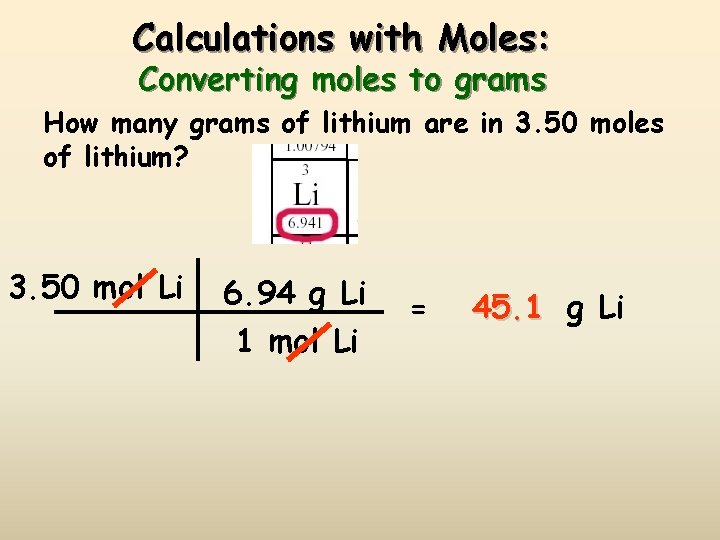
Calculations with Moles: Converting moles to grams How many grams of lithium are in 3. 50 moles of lithium? 3. 50 mol Li 6. 94 g Li 1 mol Li = 45. 1 g Li

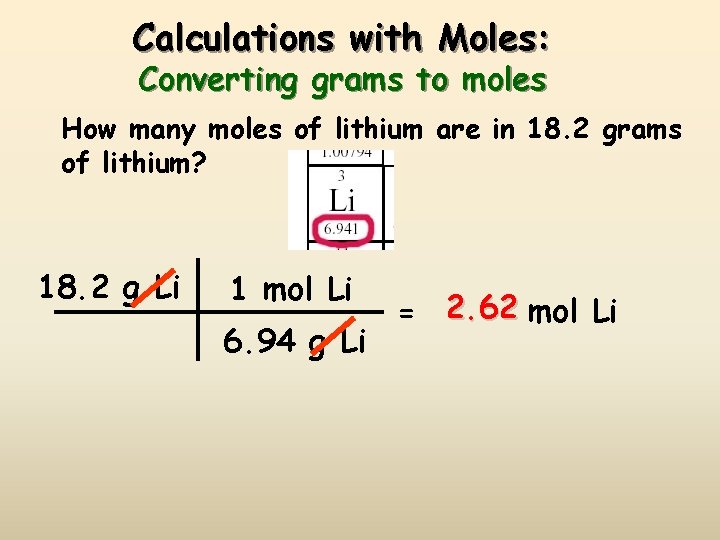
Calculations with Moles: Converting grams to moles How many moles of lithium are in 18. 2 grams of lithium? 18. 2 g Li 1 mol Li 6. 94 g Li = 2. 62 mol Li

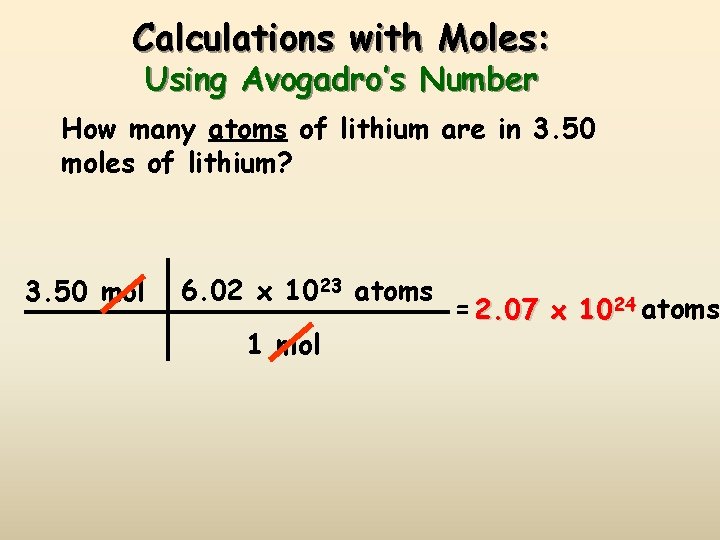
Calculations with Moles: Using Avogadro’s Number How many atoms of lithium are in 3. 50 moles of lithium? 3. 50 mol 6. 02 x 1023 atoms 1 mol = 2. 07 x 1024 atoms

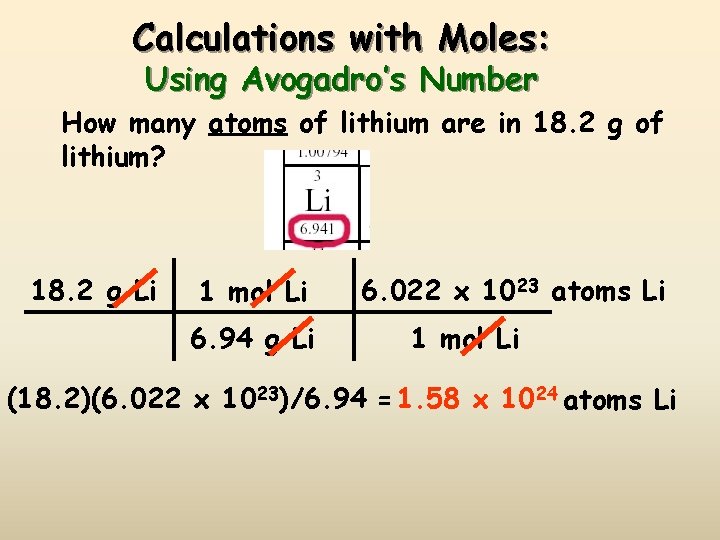
Calculations with Moles: Using Avogadro’s Number How many atoms of lithium are in 18. 2 g of lithium? 18. 2 g Li 1 mol Li 6. 94 g Li 6. 022 x 1023 atoms Li 1 mol Li (18. 2)(6. 022 x 1023)/6. 94 = 1. 58 x 1024 atoms Li