Molecules of Life What are organic molecules Compounds













- Slides: 62

Molecules of Life What are organic molecules? Compounds that contain carbon What are biological molecules? Carbohydrates Lipids Proteins Nucleic Acids

Organic Molecules What is a cell made up of mostly? Mostly water, but what else? Carbon based molecules Why is carbon so significant for these molecules?

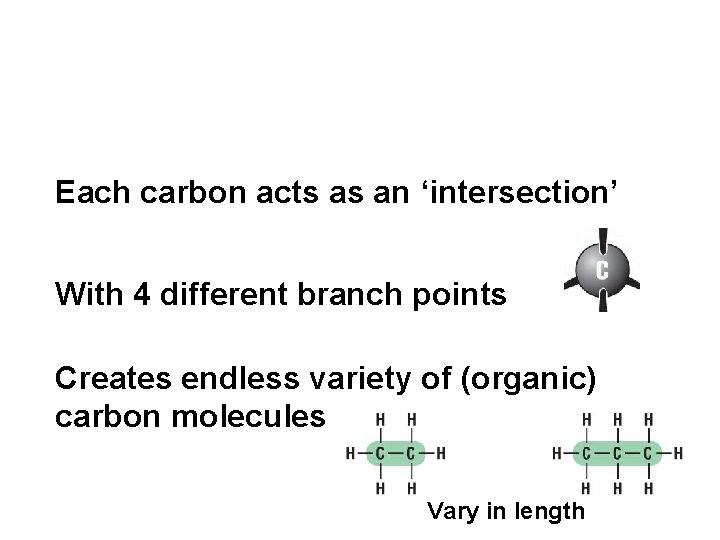
Each carbon acts as an ‘intersection’ With 4 different branch points Creates endless variety of (organic) carbon molecules Vary in length

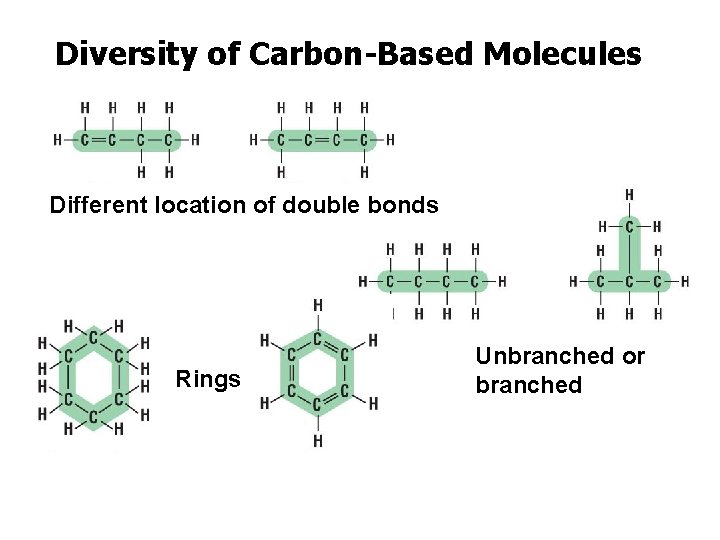
Diversity of Carbon-Based Molecules Different location of double bonds Rings Unbranched or branched

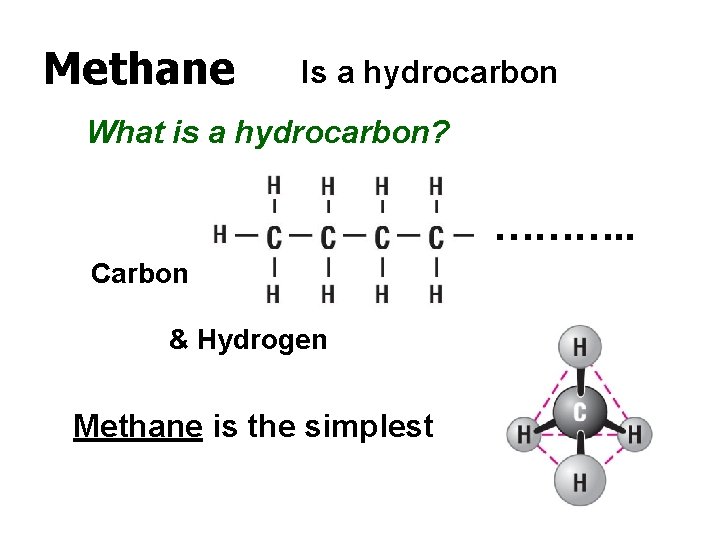
Methane Is a hydrocarbon What is a hydrocarbon? ………. . Carbon & Hydrogen Methane is the simplest

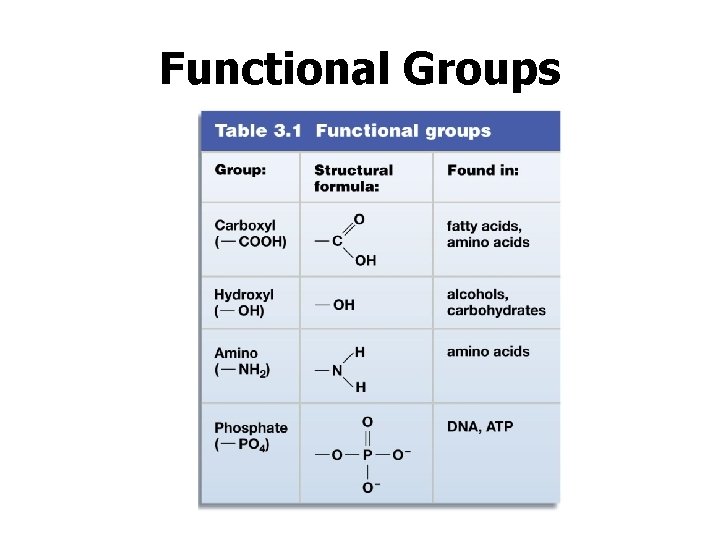
Functional Groups • Groups of atoms known as functional groups can give special properties on carbon-based molecules. • Carbon is a central element to life because most biological molecules are built on a carbon framework.

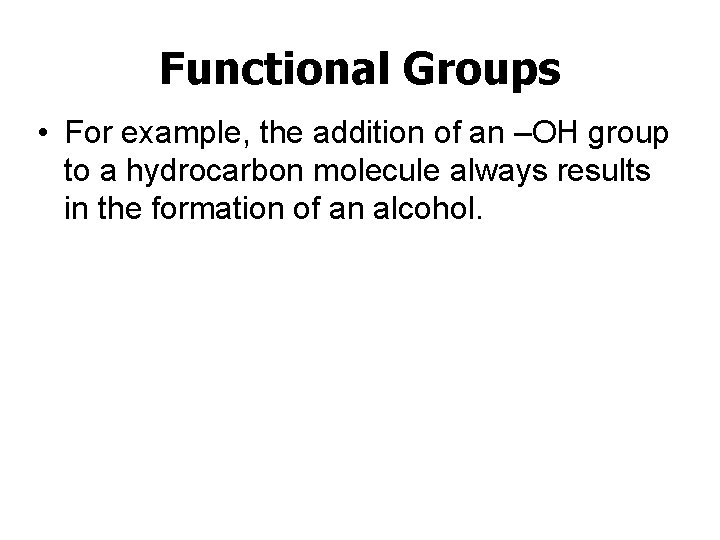
Functional Groups • For example, the addition of an –OH group to a hydrocarbon molecule always results in the formation of an alcohol.

Functional Groups

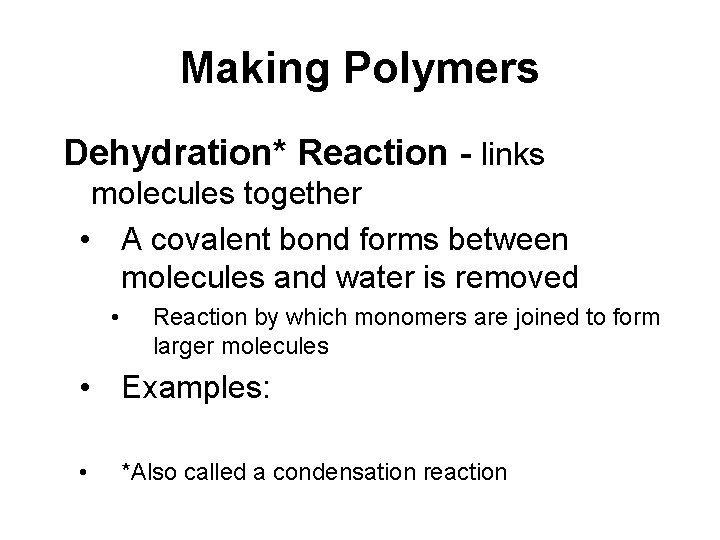
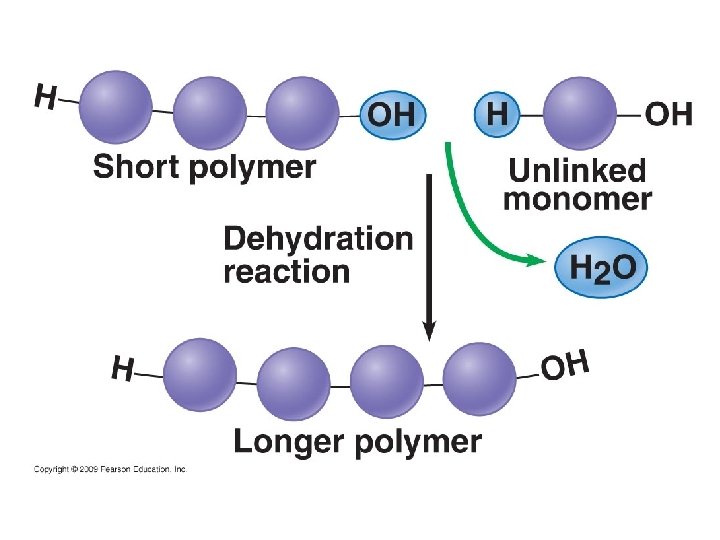
Making Polymers Dehydration* Reaction - links molecules together • A covalent bond forms between molecules and water is removed • Reaction by which monomers are joined to form larger molecules • Examples: • *Also called a condensation reaction


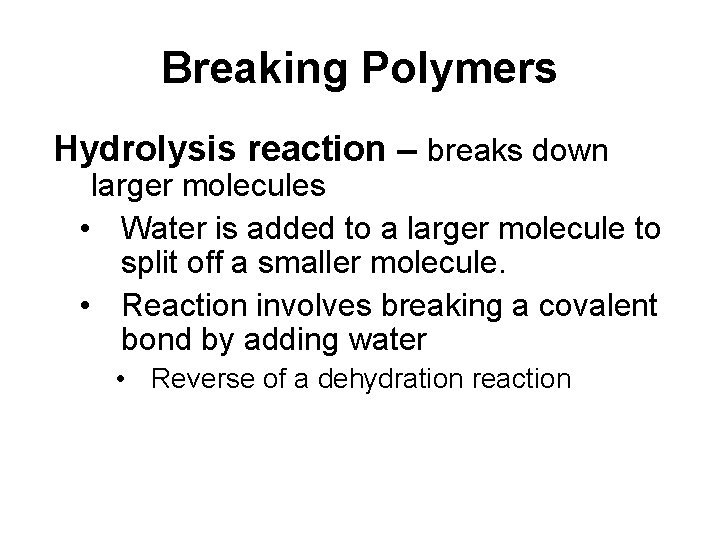
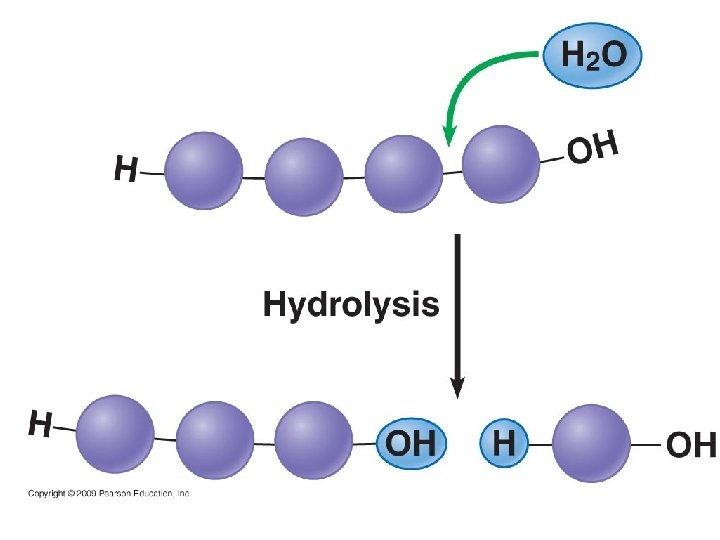
Breaking Polymers Hydrolysis reaction – breaks down larger molecules • Water is added to a larger molecule to split off a smaller molecule. • Reaction involves breaking a covalent bond by adding water • Reverse of a dehydration reaction


Biological Molecules ‘Carbs’ Sugar Glucose Glycogen Cellulose Oils Fatty acids (sat & unsat) Butter Food Structural Storage Enzymes Antibodies DNA RNA

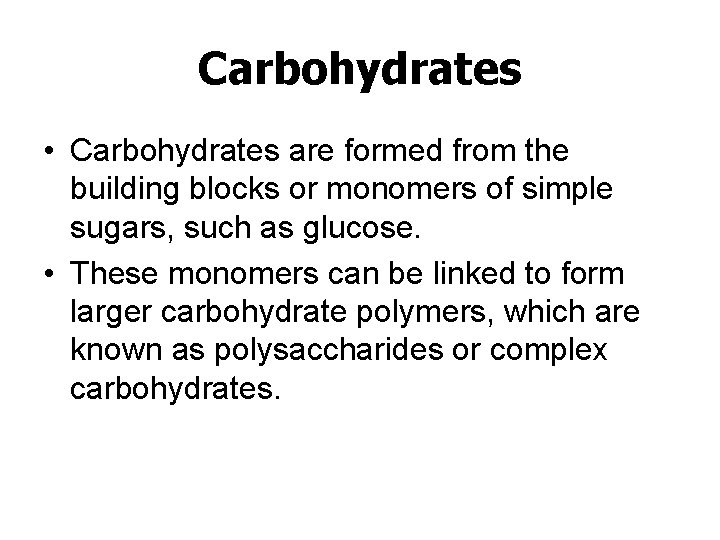
Carbohydrates • Carbohydrates are formed from the building blocks or monomers of simple sugars, such as glucose. • These monomers can be linked to form larger carbohydrate polymers, which are known as polysaccharides or complex carbohydrates.

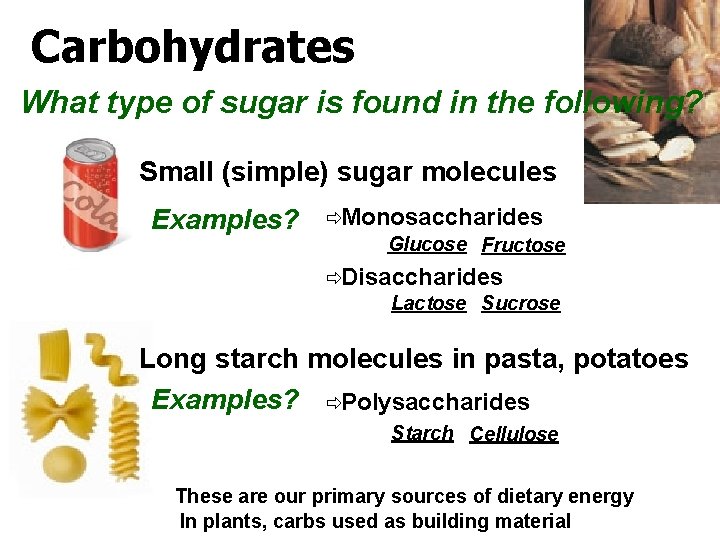
Carbohydrates What type of sugar is found in the following? Small (simple) sugar molecules Examples? ðMonosaccharides Glucose Fructose ðDisaccharides Lactose Sucrose Long starch molecules in pasta, potatoes Examples? ðPolysaccharides Starch Cellulose These are our primary sources of dietary energy In plants, carbs used as building material

Monosaccharides What type of sugar is found a sports drink? Glucose What type of sugar is found in fruit? Fructose

What about honey? Its really sweet? Why? It contains both glucose and fructose

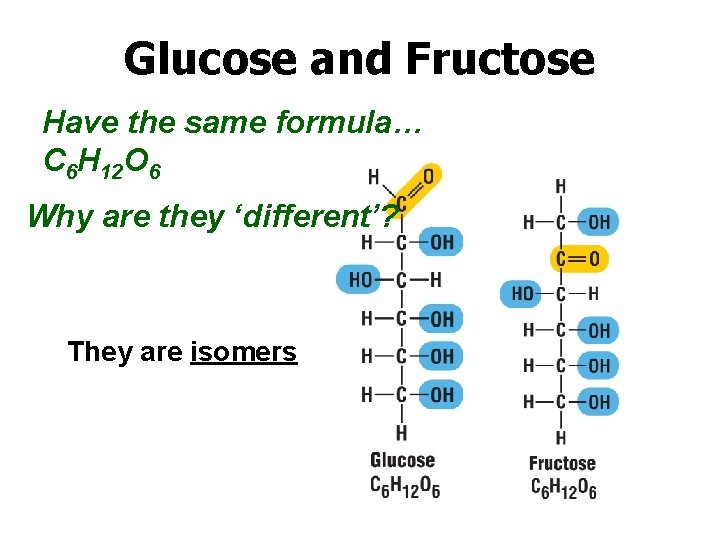
Glucose and Fructose Have the same formula… C 6 H 12 O 6 Why are they ‘different’? They are isomers

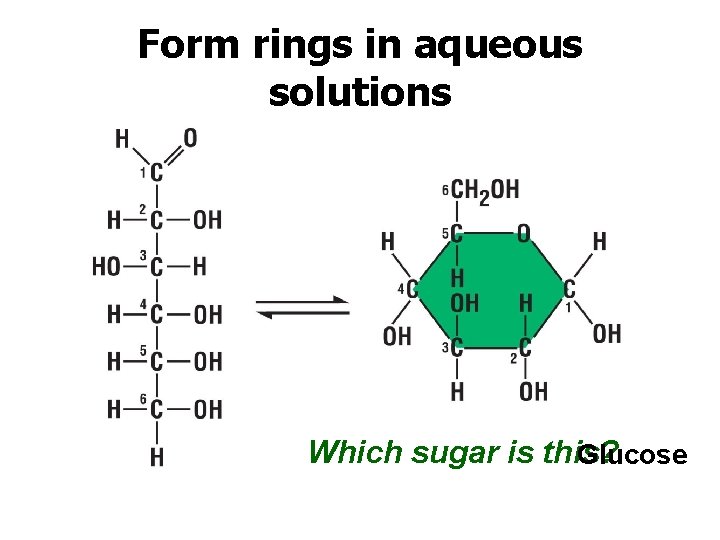
Form rings in aqueous solutions Which sugar is this? Glucose

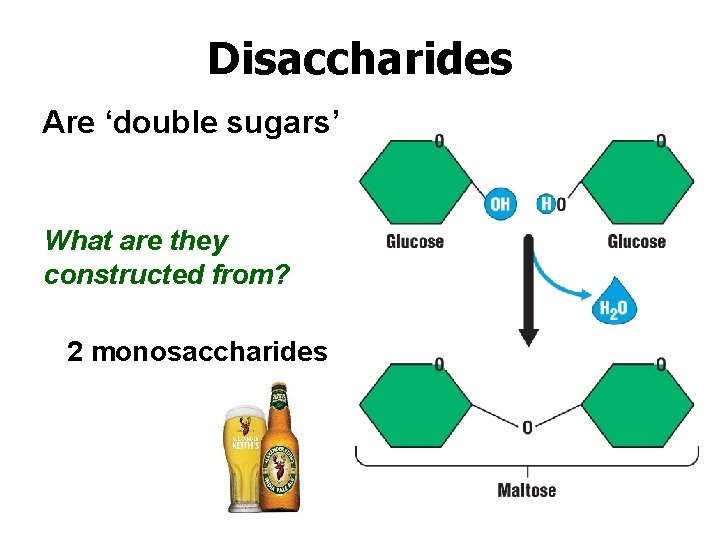
Disaccharides Are ‘double sugars’ What are they constructed from? 2 monosaccharides

Disaccharides Maltose: glucose and glucose Lactose: galactose and glucose Sucrose: glucose and fructose

Lactose, another disaccharide • Some people have trouble digesting lactose • Its a condition called lactose intolerance • Missing gene for lactase enzyme

Sucrose The most common disaccharide is sucrose, what do you know it as? Common table sugar What plants do we use to extract table sugar? Sugar cane Roots of sugar beets

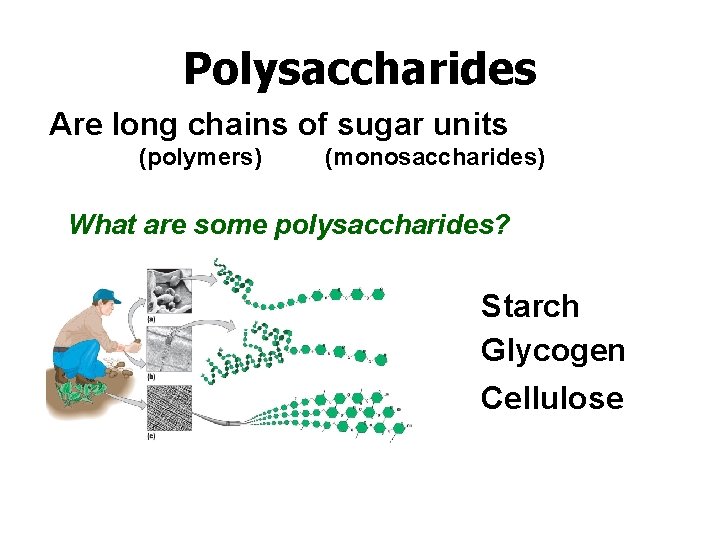
Polysaccharides Are long chains of sugar units (polymers) (monosaccharides) What are some polysaccharides? Starch Glycogen Cellulose

Starch Potatoes and grains are major sources of starch in the human diet Glycogen Liver, muscle cells break down glycogen to release glucose when needed for energy Cellulose Structural component, dietary fiber

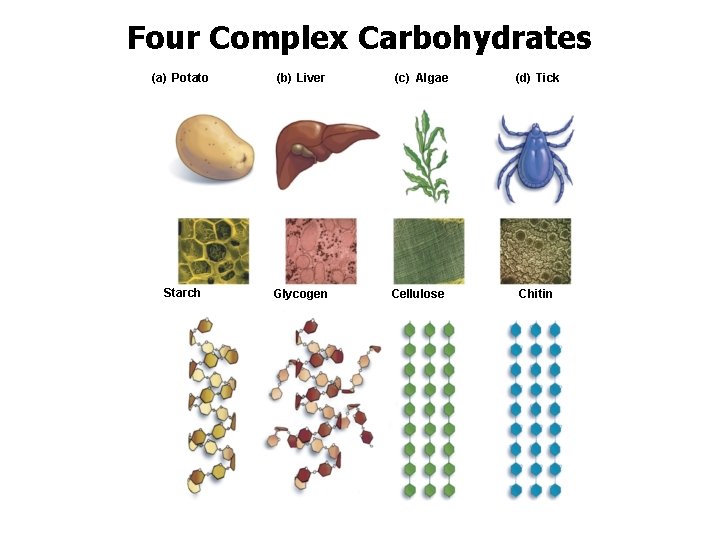
Complex Carbohydrates • Four polysaccharides are critical in the living world: – starch – glycogen – cellulose – chitin

Four Complex Carbohydrates (a) Potato (b) Liver Starch Glycogen (c) Algae Cellulose (d) Tick Chitin

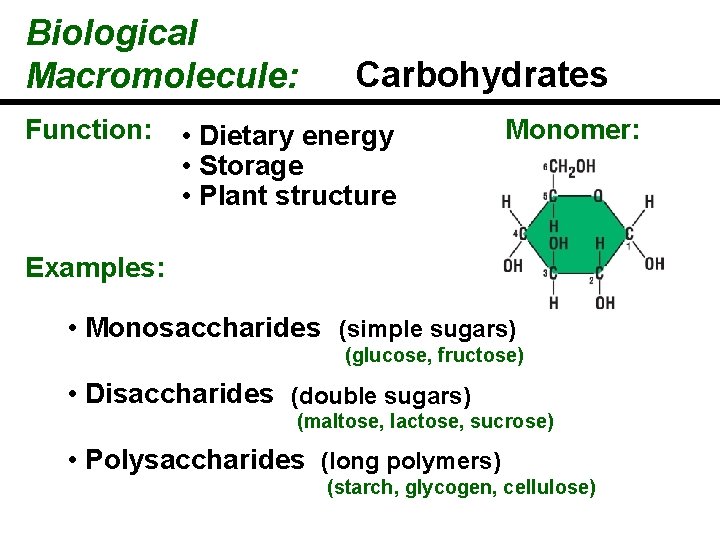
Biological Macromolecule: Function: Carbohydrates • Dietary energy • Storage • Plant structure Monomer: Examples: • Monosaccharides (simple sugars) (glucose, fructose) • Disaccharides (double sugars) (maltose, lactose, sucrose) • Polysaccharides (long polymers) (starch, glycogen, cellulose)

1. Starch is the nutrient storage form of carbohydrates in plants. 2. Glycogen is the nutrient storage form of carbohydrates in animals.

Lipids Butter, lard, margarine, and salad oil Do these lipids mix well with water? Lipids do not possess the monomers-to-polymers structure seen in other biological molecules; no one structural element is common to all lipids. Among the most important lipids are the triglycerides, composed of a glyceride and three fatty acids. Most of the fats that human beings consume are triglycerides.

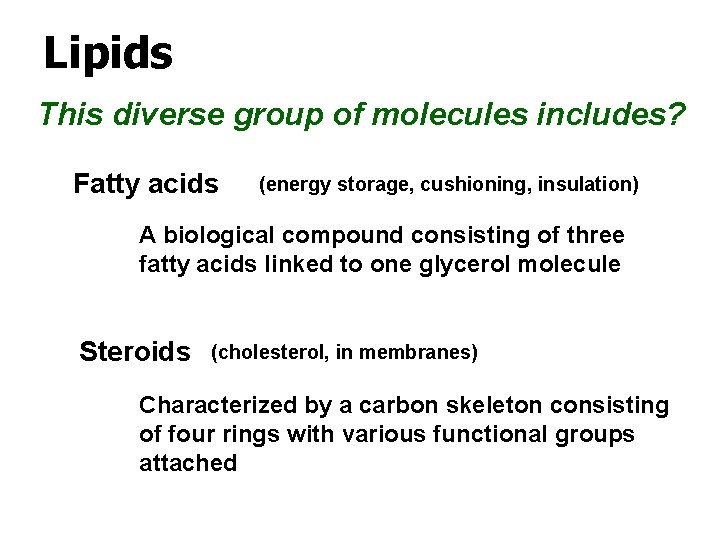
Lipids This diverse group of molecules includes? Fatty acids (energy storage, cushioning, insulation) A biological compound consisting of three fatty acids linked to one glycerol molecule Steroids (cholesterol, in membranes) Characterized by a carbon skeleton consisting of four rings with various functional groups attached

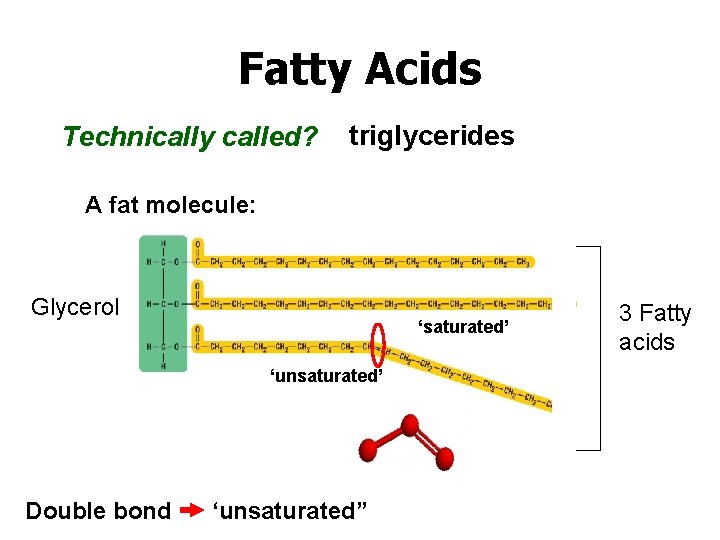
Fatty Acids Technically called? triglycerides A fat molecule: Glycerol ‘saturated’ ‘unsaturated’ Double bond ‘unsaturated” 3 Fatty acids

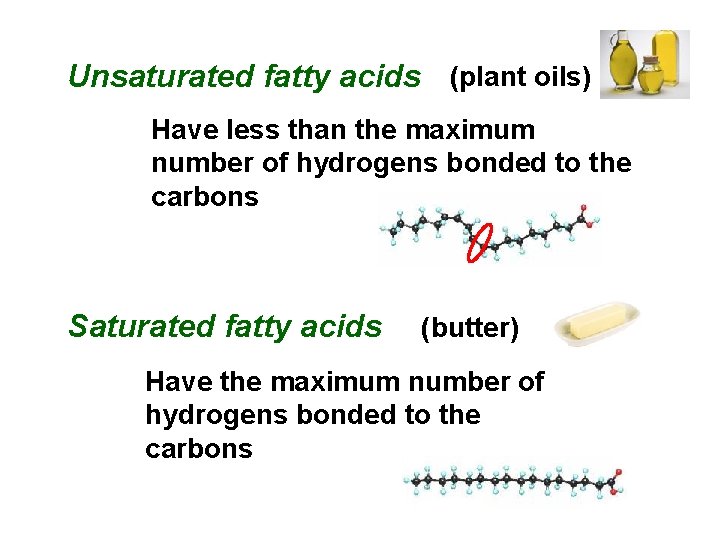
Unsaturated fatty acids (plant oils) Have less than the maximum number of hydrogens bonded to the carbons Saturated fatty acids (butter) Have the maximum number of hydrogens bonded to the carbons

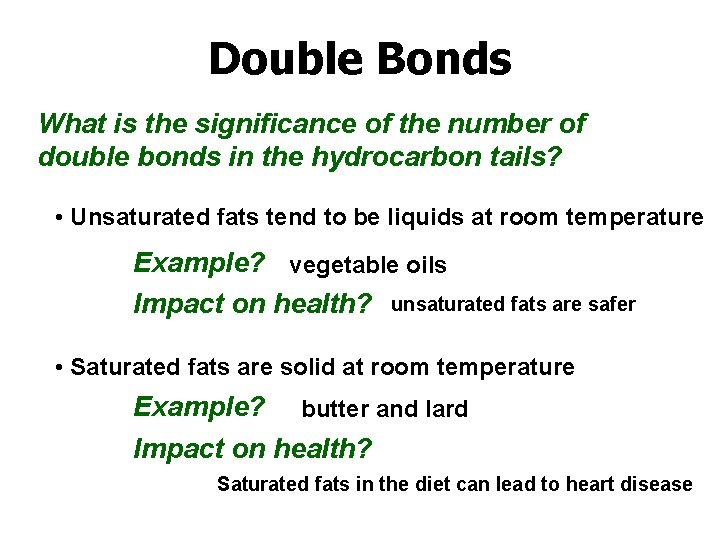
Double Bonds What is the significance of the number of double bonds in the hydrocarbon tails? • Unsaturated fats tend to be liquids at room temperature Example? vegetable oils Impact on health? unsaturated fats are safer • Saturated fats are solid at room temperature Example? butter and lard Impact on health? Saturated fats in the diet can lead to heart disease

Steroids How does the structure differ from fatty acids? Ring structure, various functional groups How does the function differ from fatty acids? Functional groups affect function Example? • causes differences between the hormones estrogen and testosterone (anatomical and physical development) • cholesterol in membranes

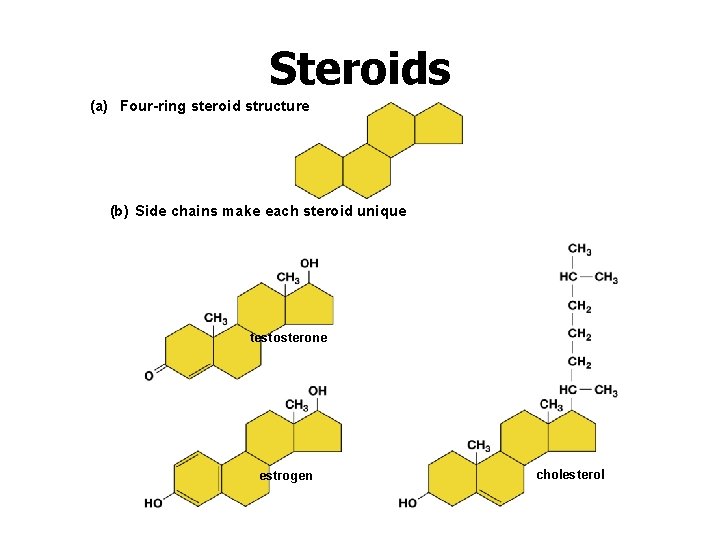
Steroids (a) Four-ring steroid structure (b) Side chains make each steroid unique testosterone estrogen cholesterol

Phospholipids • A third class of lipids is the phospholipids, each of which is composed of two fatty acids, glycerol, and a phosphate group. • The material forming the outer membrane of cells is largely composed of phospholipids.

Waxes • A fourth class of lipids is the waxes, each of which is composed of a single fatty acid linked to a long-chain alcohol. • Waxes have an important “sealing” function in the living world. • Almost all plant surfaces exposed to air, for example, have a protective covering made largely of wax.

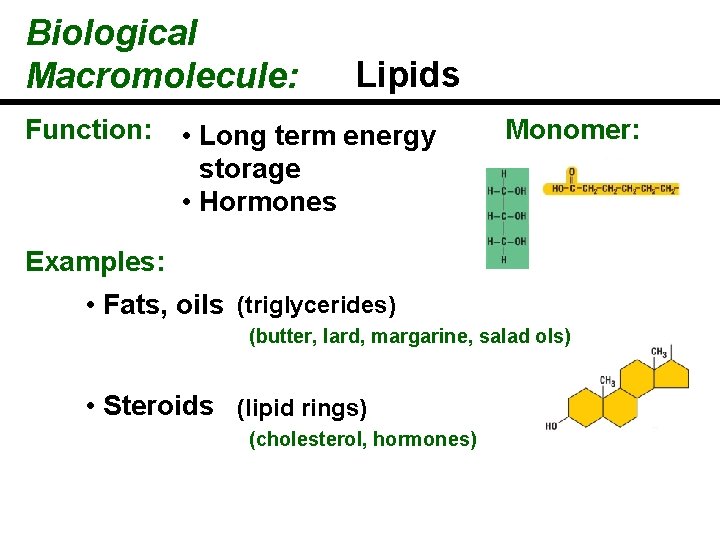
Biological Macromolecule: Function: Lipids • Long term energy storage • Hormones Monomer: Examples: • Fats, oils (triglycerides) (butter, lard, margarine, salad ols) • Steroids (lipid rings) (cholesterol, hormones)

Proteins What is a protein? Proteins are an extremely diverse group of biological molecules composed of the monomers called amino acids. • Constructed from a set of 20 different monomers

Proteins • Sequences of amino acids are strung together to produce polypeptide chains, which then fold up into working proteins. • Important groups of proteins include enzymes, which hasten chemical reactions, and structural proteins, which make up such structures as hair.

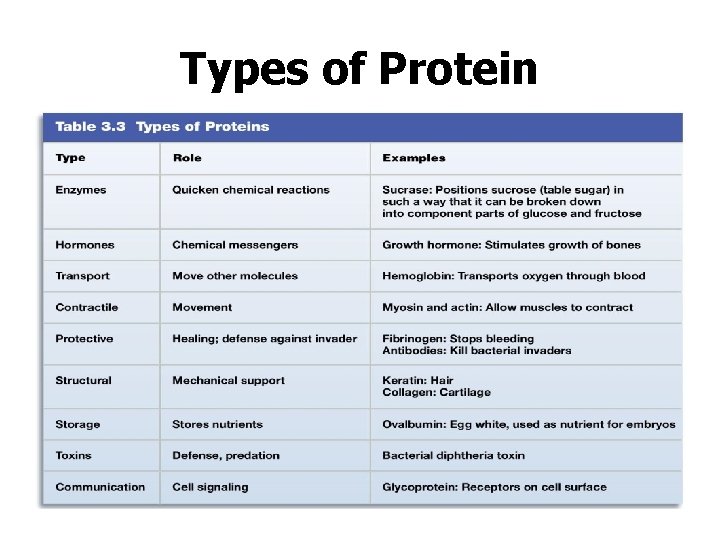
Types of Protein

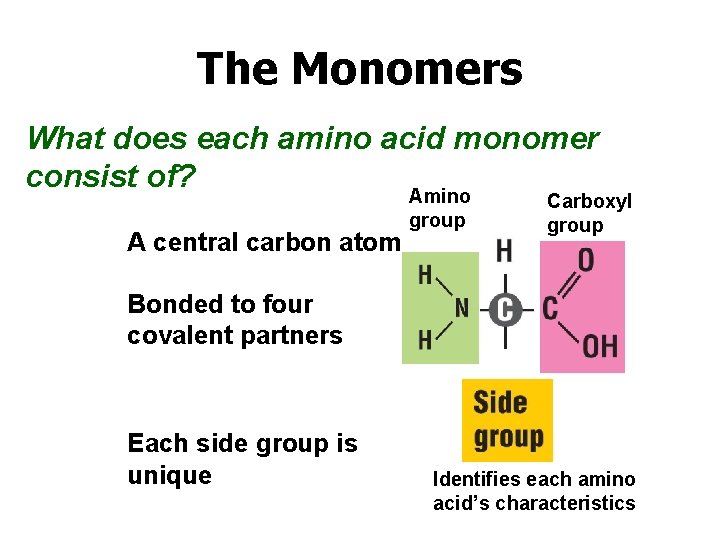
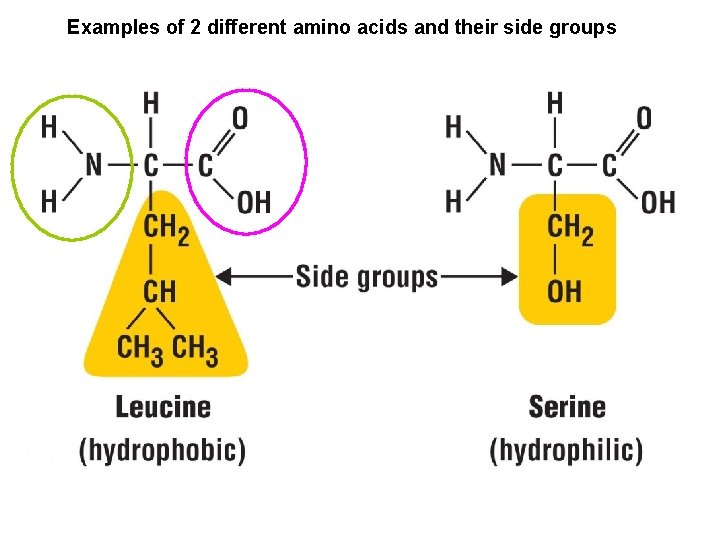
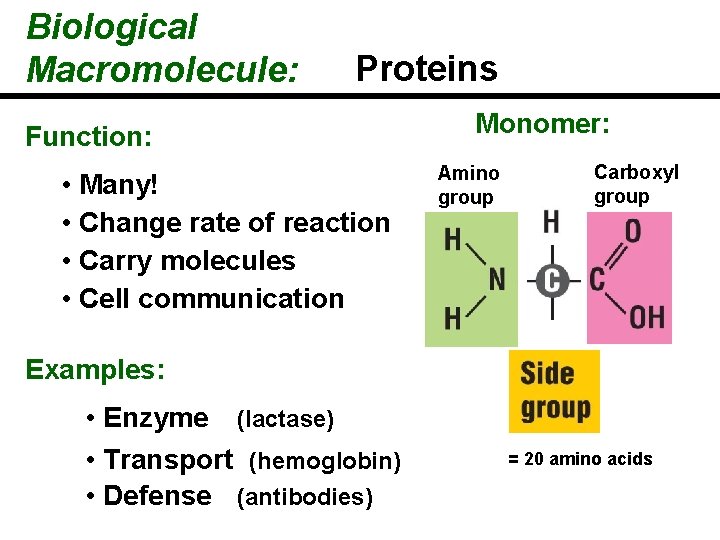
The Monomers What does each amino acid monomer consist of? Amino A central carbon atom group Carboxyl group Bonded to four covalent partners Each side group is unique Identifies each amino acid’s characteristics

Examples of 2 different amino acids and their side groups

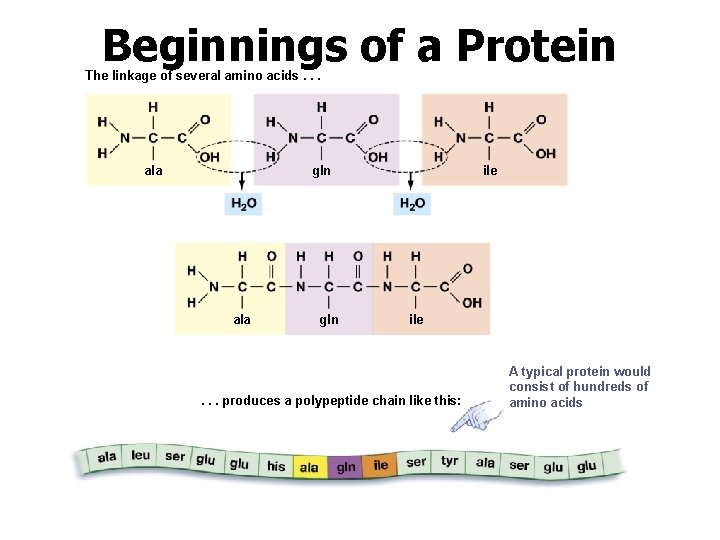
Beginnings of a Protein The linkage of several amino acids. . . ala gln ile . . . produces a polypeptide chain like this: A typical protein would consist of hundreds of amino acids

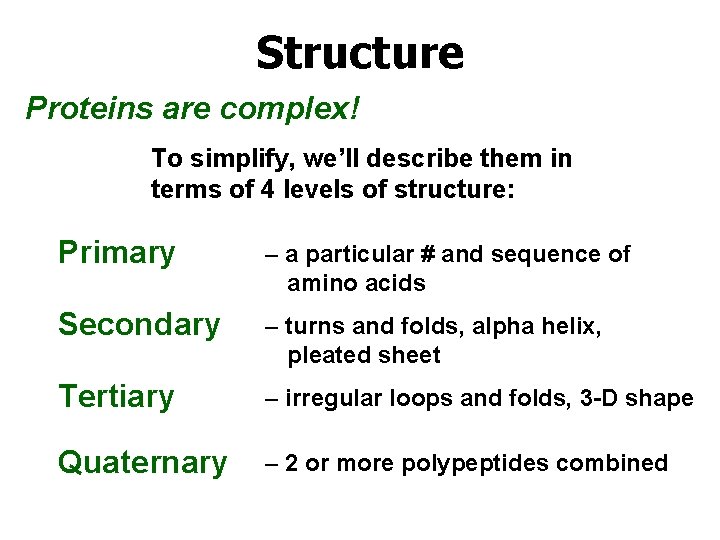
Structure Proteins are complex! To simplify, we’ll describe them in terms of 4 levels of structure: Primary – a particular # and sequence of amino acids Secondary – turns and folds, alpha helix, pleated sheet Tertiary – irregular loops and folds, 3 -D shape Quaternary – 2 or more polypeptides combined

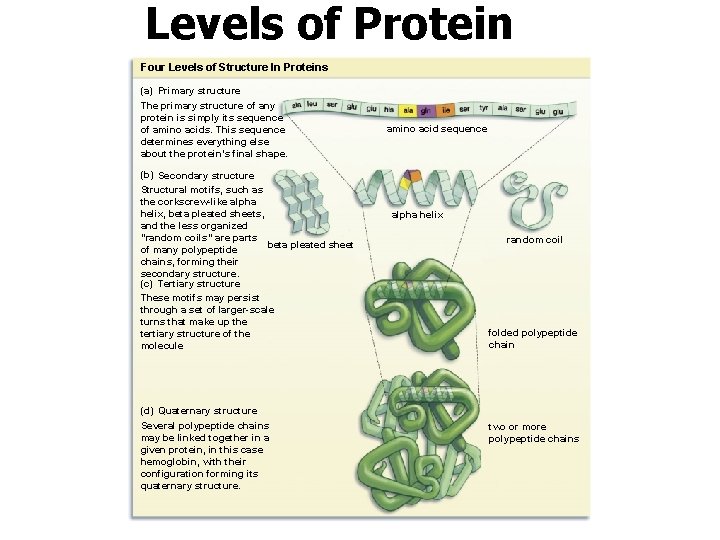
Levels of Protein Structure Four Levels of Structure In Proteins (a) Primary structure The primary structure of any protein is simply its sequence of amino acids. This sequence determines everything else about the protein’s final shape. (b) Secondary structure Structural motifs, such as the corkscrew-like alpha helix, beta pleated sheets, and the less organized “random coils” are parts beta pleated sheet of many polypeptide chains, forming their secondary structure. (c) Tertiary structure These motifs may persist through a set of larger-scale turns that make up the tertiary structure of the molecule (d) Quaternary structure Several polypeptide chains may be linked together in a given protein, in this case hemoglobin, with their configuration forming its quaternary structure. amino acid sequence alpha helix random coil folded polypeptide chain two or more polypeptide chains

Biological Macromolecule: Proteins Function: • Many! • Change rate of reaction • Carry molecules • Cell communication Monomer: Amino group Carboxyl group Examples: • Enzyme (lactase) • Transport (hemoglobin) • Defense (antibodies) = 20 amino acids

Biological Molecules

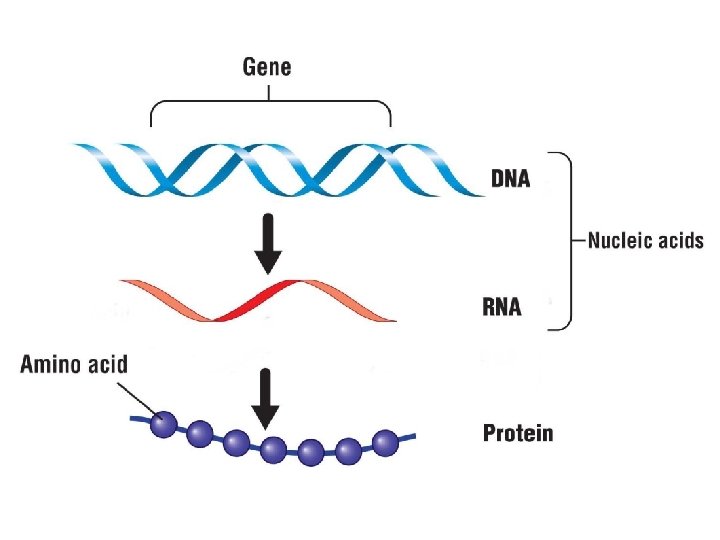
Nucleic Acids What are nucleic acids? The cells information storage molecules • There are two types of nucleic acids DNA, deoxyribonucleic acid RNA, ribonucleic acid • These ‘work together’ to synthesize protein

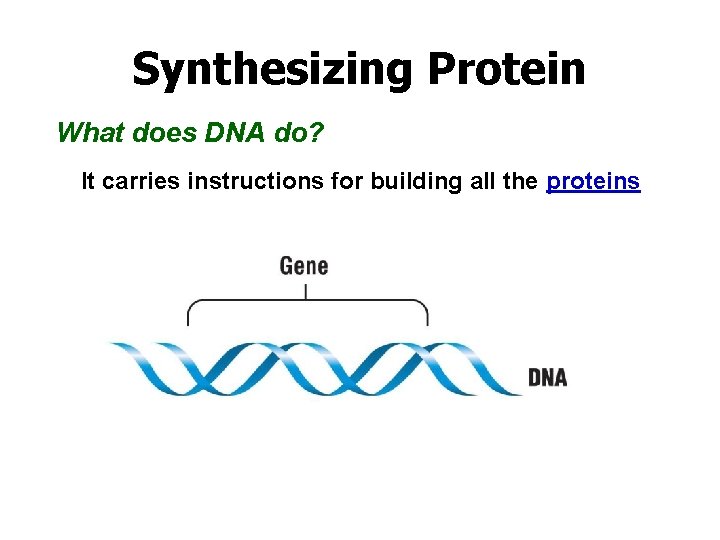
Synthesizing Protein What does DNA do? It carries instructions for building all the proteins

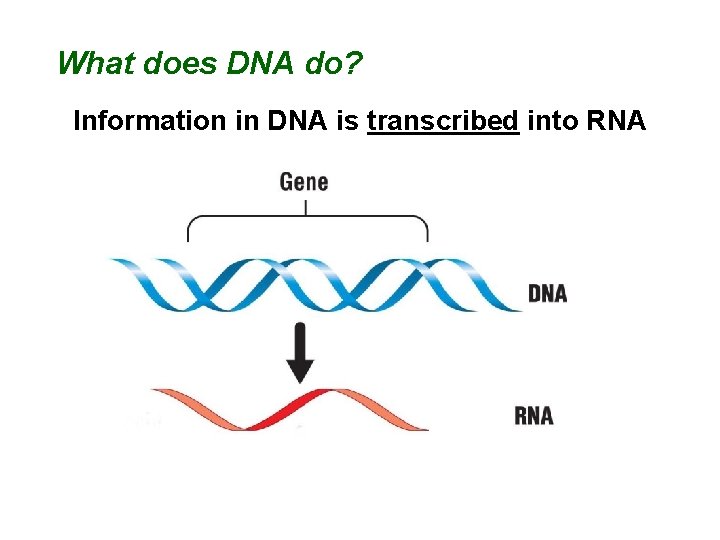
What does DNA do? Information in DNA is transcribed into RNA

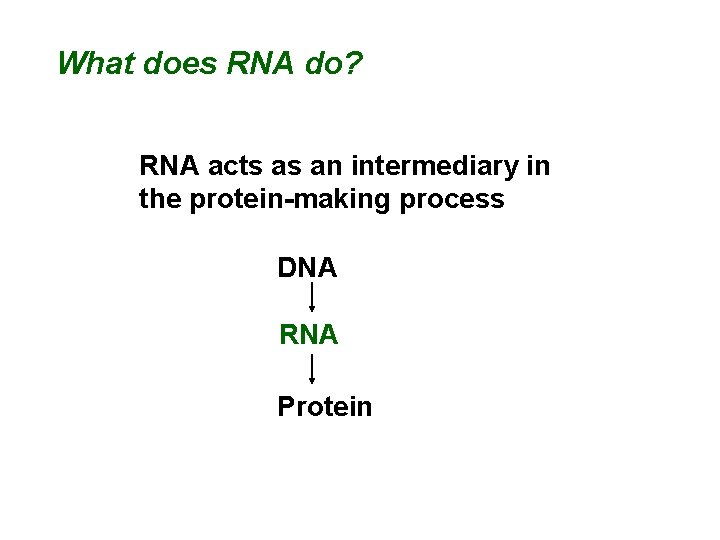
What does RNA do? RNA acts as an intermediary in the protein-making process DNA RNA Protein

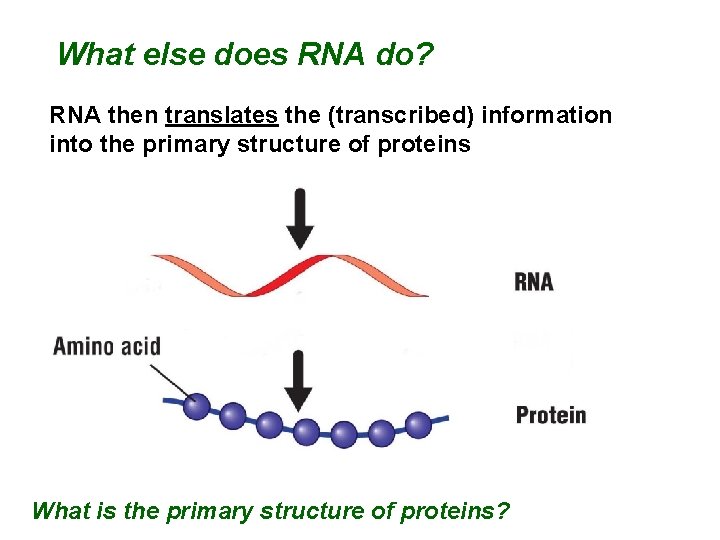
What else does RNA do? RNA then translates the (transcribed) information into the primary structure of proteins What is the primary structure of proteins?


What does protein do? Proteins carry out cell activities

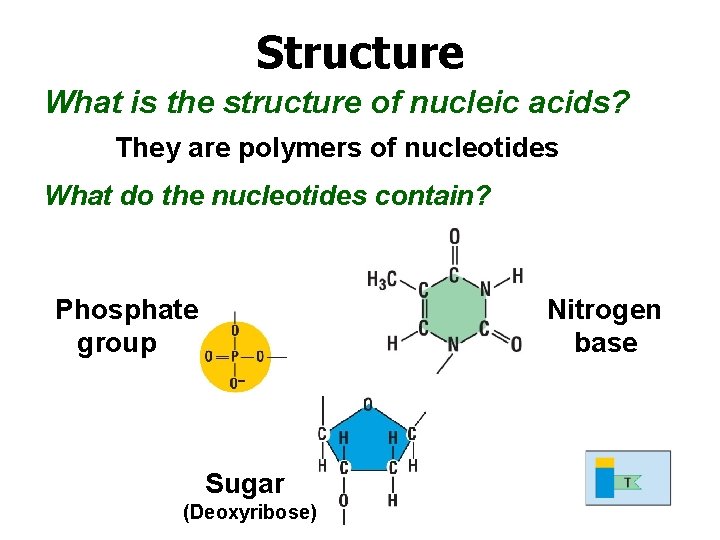
Structure What is the structure of nucleic acids? They are polymers of nucleotides What do the nucleotides contain? Phosphate group Nitrogen base Sugar (Deoxyribose)

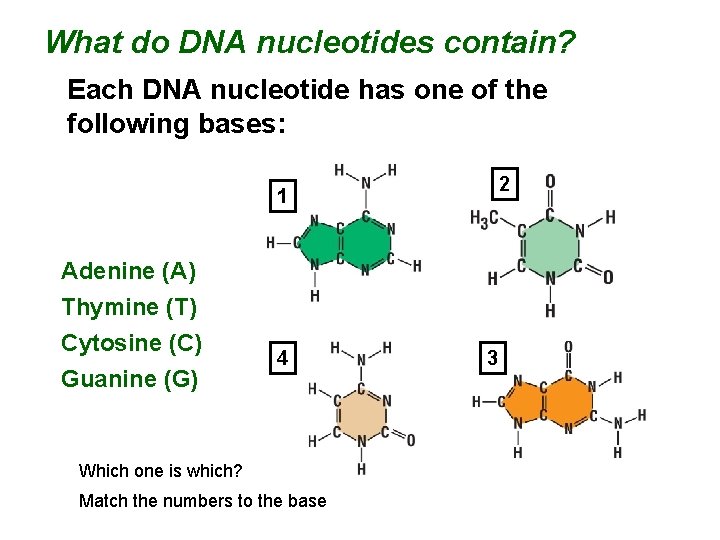
What do DNA nucleotides contain? Each DNA nucleotide has one of the following bases: 2 1 Adenine (A) Thymine (T) Cytosine (C) Guanine (G) 4 Which one is which? Match the numbers to the base 3

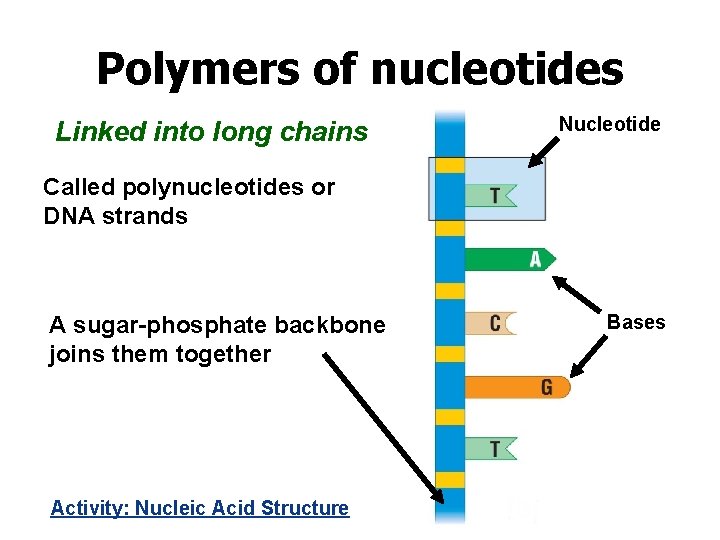
Polymers of nucleotides Linked into long chains Nucleotide Called polynucleotides or DNA strands A sugar-phosphate backbone joins them together Activity: Nucleic Acid Structure Bases

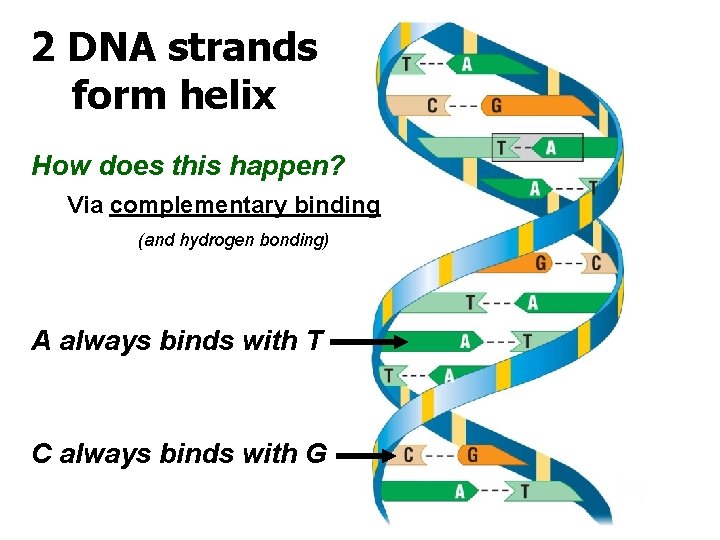
2 DNA strands form helix How does this happen? Via complementary binding (and hydrogen bonding) A always binds with T C always binds with G

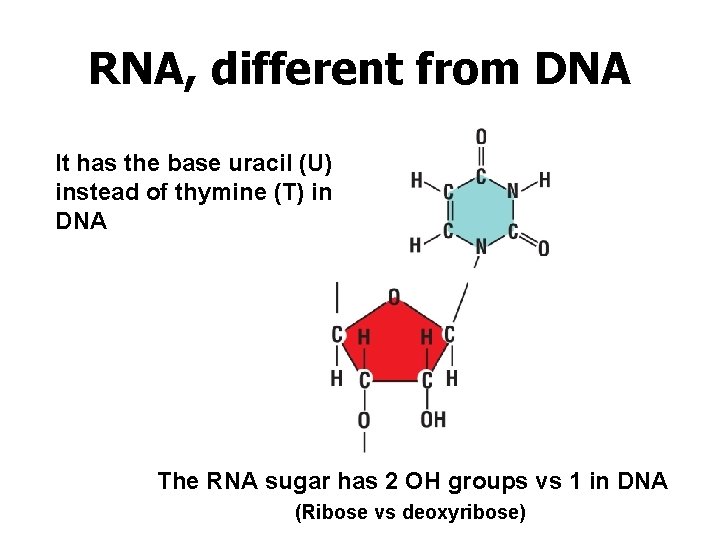
RNA, different from DNA It has the base uracil (U) instead of thymine (T) in DNA The RNA sugar has 2 OH groups vs 1 in DNA (Ribose vs deoxyribose)

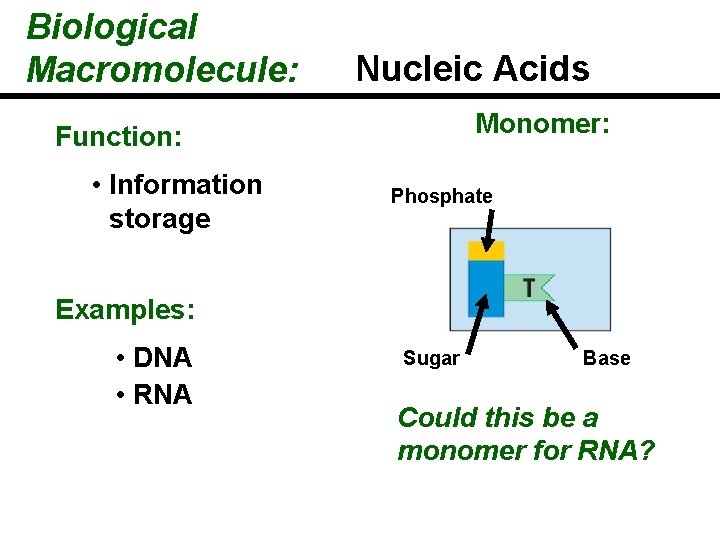
Biological Macromolecule: Nucleic Acids Monomer: Function: • Information storage Phosphate Examples: • DNA • RNA Sugar Base Could this be a monomer for RNA?