Molecules of Life Chapter 22 Great Idea A










- Slides: 23

Molecules of Life Chapter 22 Great Idea: A cell’s major parts are constructed from a few simple molecular building blocks

Chapter Outline • Organic Molecules • Proteins: The Workhorses of Life • Carbohydrates • Lipids • Minerals and Vitamins

Organic Molecules

Four Basic Characteristics • Most molecules based on chemistry of carbon – Organic molecules • Life’s molecules form from few elements – H, O, C, N 97. 5% of body weight • Molecules composed of simple building blocks – Arranged differently • Shape determines behavior – Determines ability for bonding

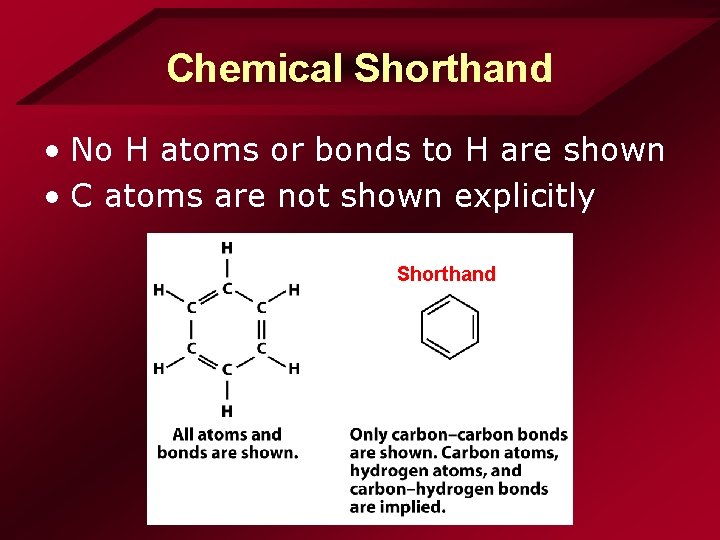
Chemical Shorthand • No H atoms or bonds to H are shown • C atoms are not shown explicitly Shorthand

Proteins: The Workhorses of Life

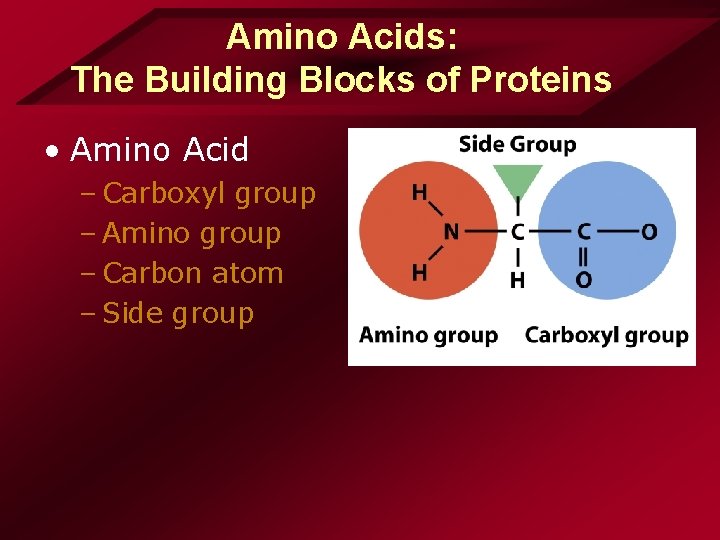
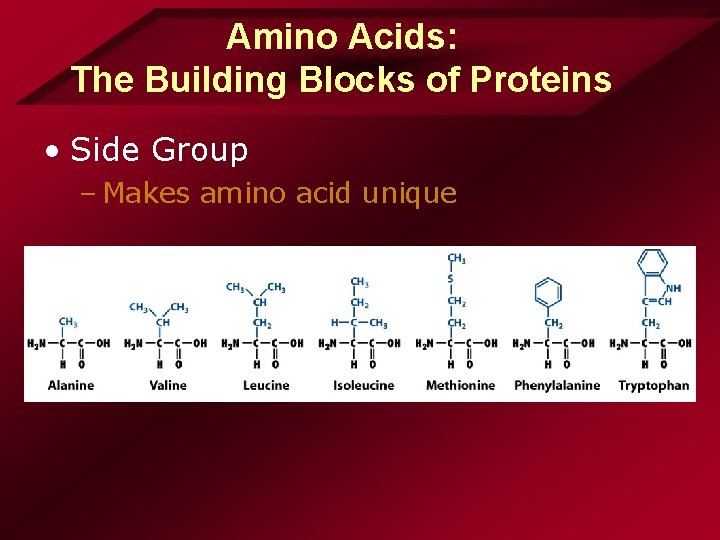
Amino Acids: The Building Blocks of Proteins • Amino Acid – Carboxyl group – Amino group – Carbon atom – Side group

Amino Acids: The Building Blocks of Proteins • Side Group – Makes amino acid unique

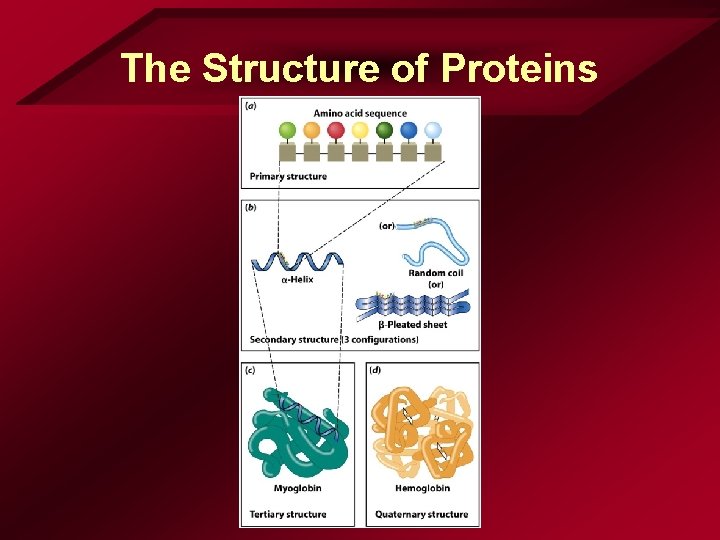
Amino Acids cont. • Bonding – Two amino acids • H bonds with OH • Forms H 2 O • Forms peptide bond – Polypeptide • chain • Protein – Large molecule – Chain of amino acids • Only 20 amino acids in living organisms

The Structure of Proteins

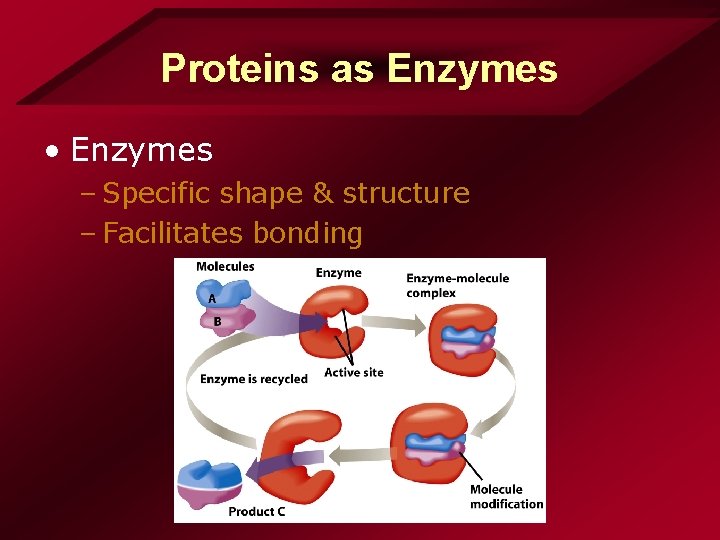
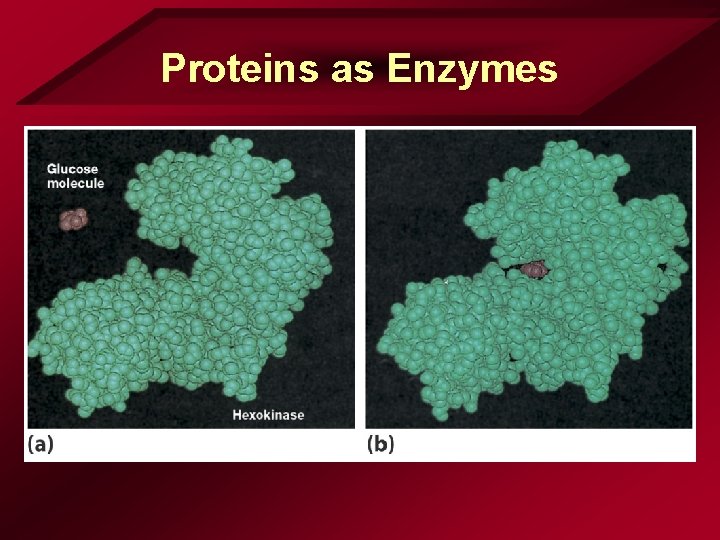
Proteins as Enzymes • Enzymes – Specific shape & structure – Facilitates bonding

Proteins as Enzymes

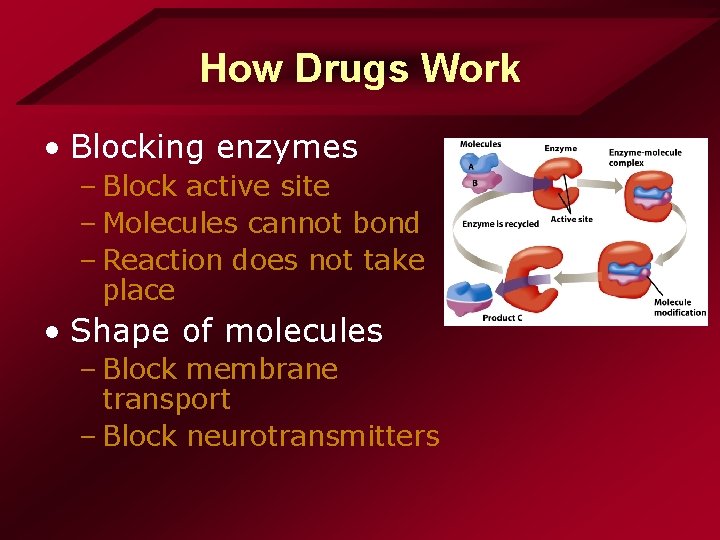
How Drugs Work • Blocking enzymes – Block active site – Molecules cannot bond – Reaction does not take place • Shape of molecules – Block membrane transport – Block neurotransmitters

Carbohydrates

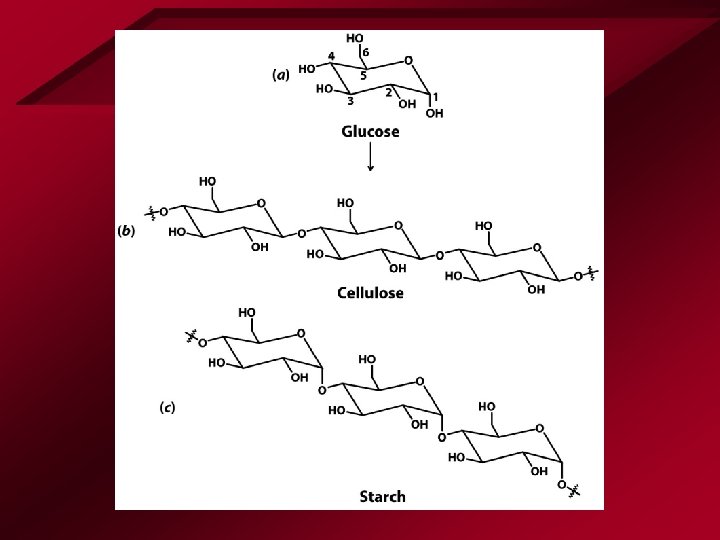
Carbohydrates • Structure – C, H, O • Simplest – Sugars – Cn. H 2 n. On • • Monosaccharides Polysaccharide Starches Cellulose


Lipids

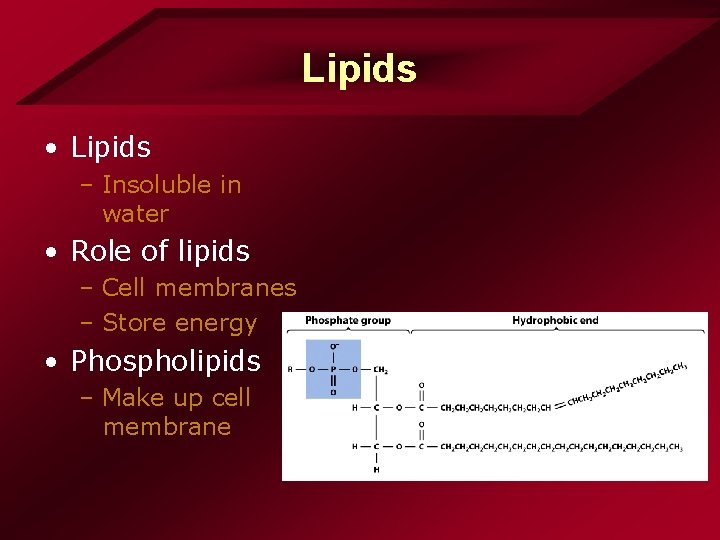
Lipids • Lipids – Insoluble in water • Role of lipids – Cell membranes – Store energy • Phospholipids – Make up cell membrane

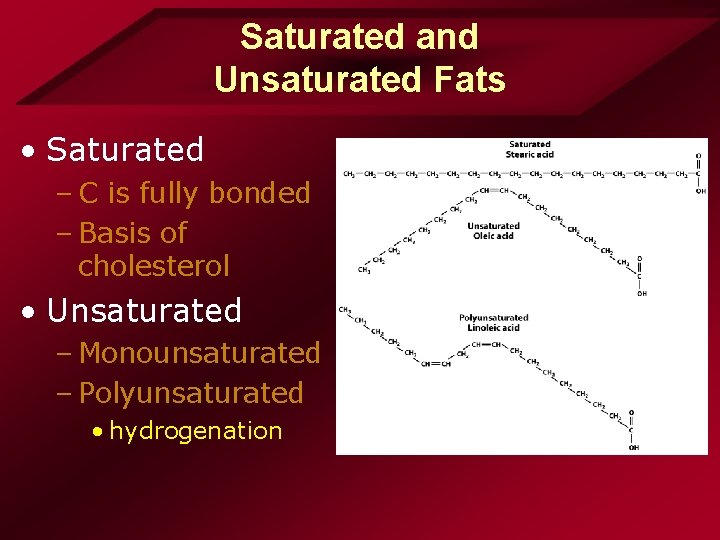
Saturated and Unsaturated Fats • Saturated – C is fully bonded – Basis of cholesterol • Unsaturated – Monounsaturated – Polyunsaturated • hydrogenation

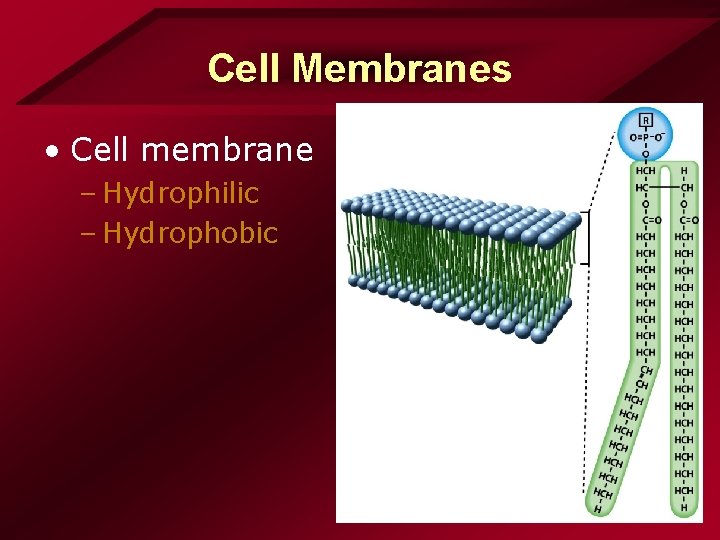
Cell Membranes • Cell membrane – Hydrophilic – Hydrophobic

Minerals and Vitamins

Minerals • Minerals – All chemical elements except C, H, N, O • Example – Calcium • 2% of weight

Vitamins • Vitamins – Organic molecules – Must be taken in with food • Except vitamin D • Water soluble – Vitamins B & C • Fat soluble – Vitamins A, D, E, & K • Function – Assist enzymes