Molecules Chemical Bonds Packet 4 Chapter 2 Friday

Molecules & Chemical Bonds Packet #4 Chapter #2 Friday, December 31, 2021 1

Introduction �Compound �A substance consisting of two or more elements in a fixed ratio �Molecule �Two or more atoms held together by covalent bonds. �Chemical Bonds �Forces by which atoms are able to bond together. �An attraction between two atoms � Results from the sharing of electrons. � Results from the presence of opposite charges. Friday, December 31, 2021 2

Molecules Chemical Formulas Friday, December 31, 2021 3

Introduction II �Molecular Formulas �Says what the molecule is made of and how many they are �Structural Formula �Shows how the molecule is orientated in space 12/31/2021 4: 26: 42 AM 4
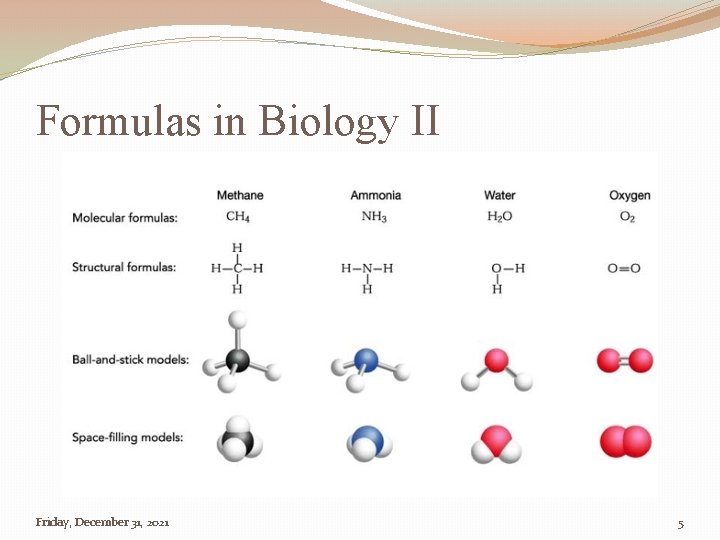
Formulas in Biology II Friday, December 31, 2021 5

Types of Chemical Bonds �Covalent Bond �Ionic Bond �Hydrogen Bond �Polar Bond �Non-polar Bond 12/31/2021 4: 26: 46 AM 6

Types of Chemical Bonds Covalent Bonds Friday, December 31, 2021 7
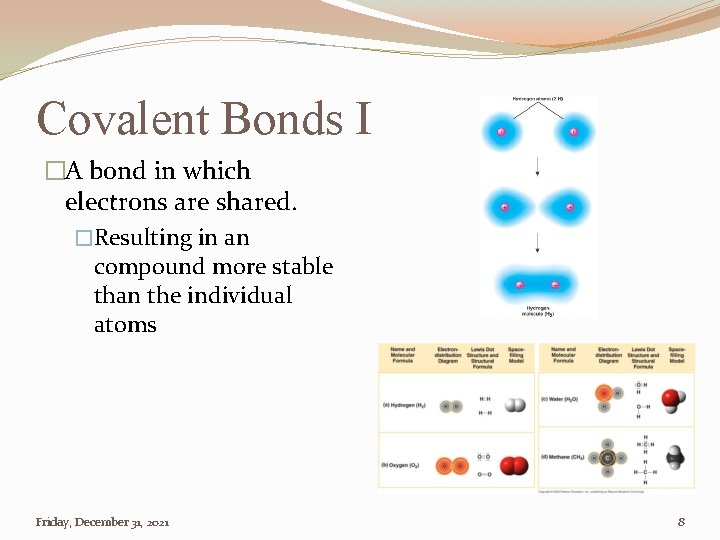
Covalent Bonds I �A bond in which electrons are shared. �Resulting in an compound more stable than the individual atoms Friday, December 31, 2021 8
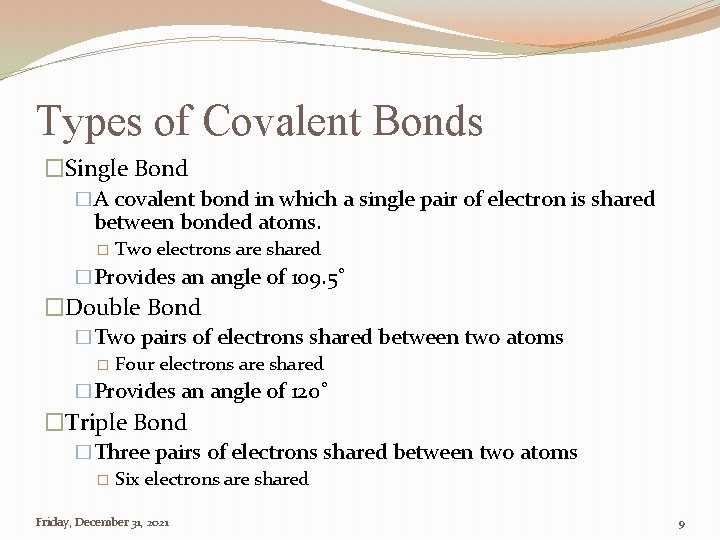
Types of Covalent Bonds �Single Bond �A covalent bond in which a single pair of electron is shared between bonded atoms. � Two electrons are shared �Provides an angle of 109. 5˚ �Double Bond �Two pairs of electrons shared between two atoms � Four electrons are shared �Provides an angle of 120˚ �Triple Bond �Three pairs of electrons shared between two atoms � Six electrons are shared Friday, December 31, 2021 9
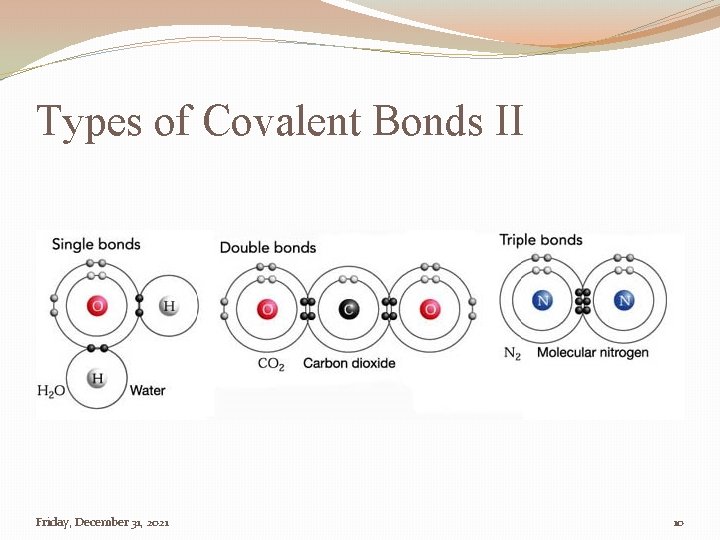
Types of Covalent Bonds II Friday, December 31, 2021 10
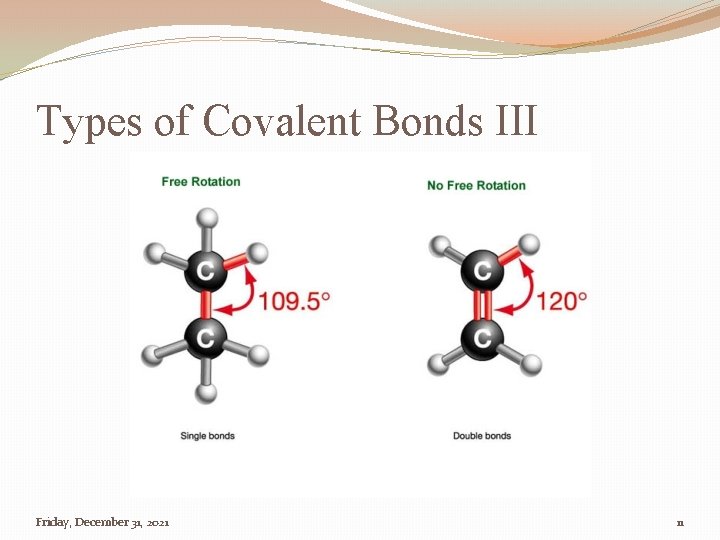
Types of Covalent Bonds III Friday, December 31, 2021 11
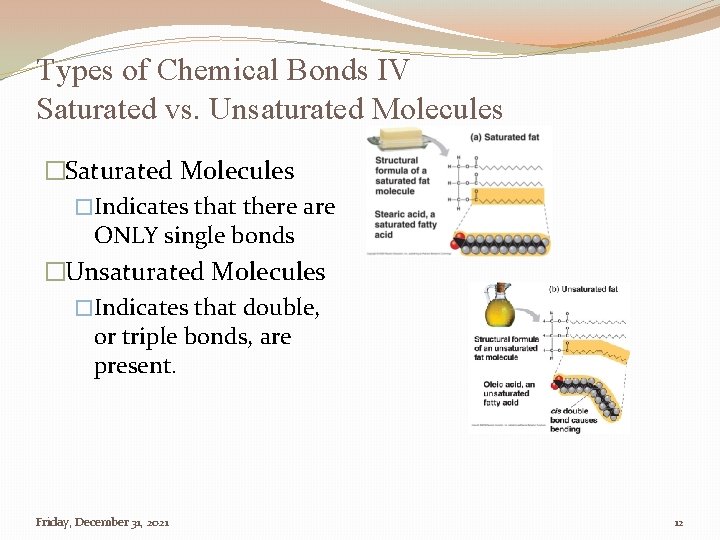
Types of Chemical Bonds IV Saturated vs. Unsaturated Molecules �Saturated Molecules �Indicates that there are ONLY single bonds �Unsaturated Molecules �Indicates that double, or triple bonds, are present. Friday, December 31, 2021 12
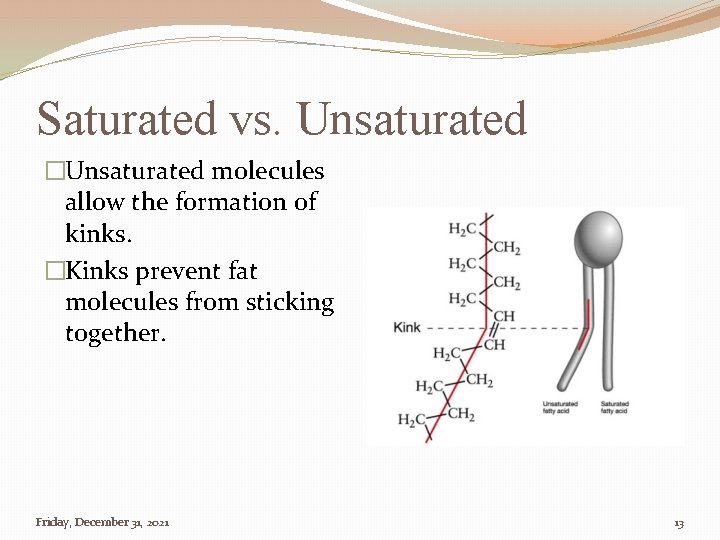
Saturated vs. Unsaturated �Unsaturated molecules allow the formation of kinks. �Kinks prevent fat molecules from sticking together. Friday, December 31, 2021 13

Types of Chemical Bonds Ionic Bonds Friday, December 31, 2021 14
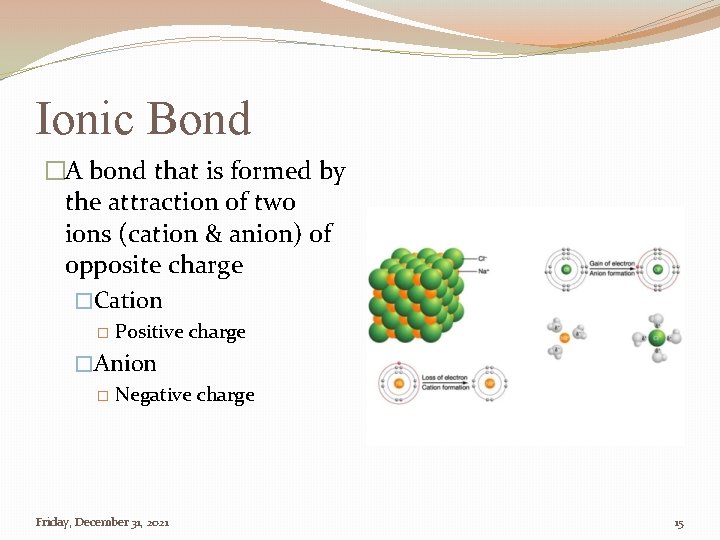
Ionic Bond �A bond that is formed by the attraction of two ions (cation & anion) of opposite charge �Cation � Positive charge �Anion � Negative charge Friday, December 31, 2021 15
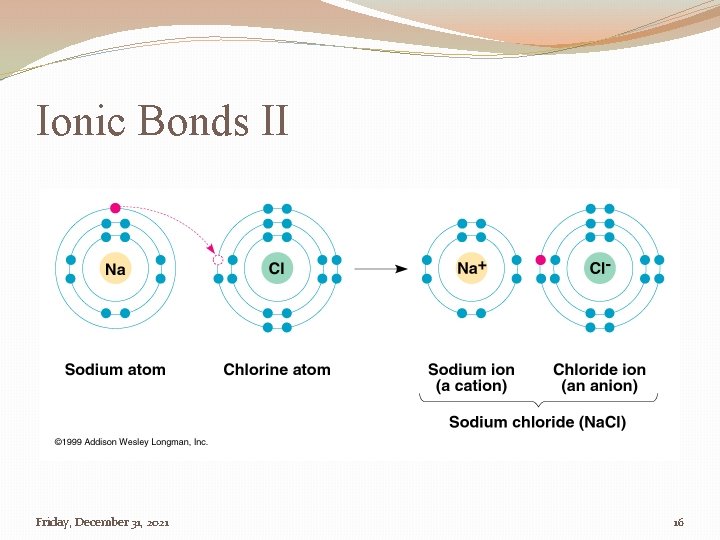
Ionic Bonds II Friday, December 31, 2021 16

Types of Chemical Bonds Polar Bond Friday, December 31, 2021 17
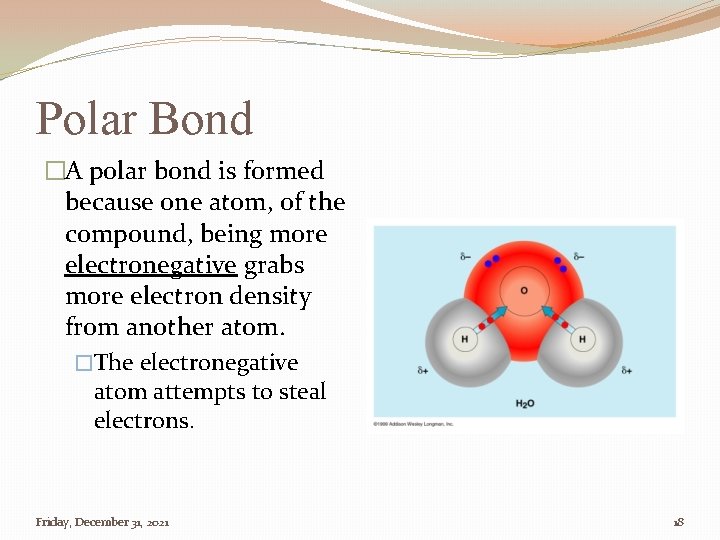
Polar Bond �A polar bond is formed because one atom, of the compound, being more electronegative grabs more electron density from another atom. �The electronegative atom attempts to steal electrons. Friday, December 31, 2021 18

Types of Chemical Bonds Non-Polar Bonds Friday, December 31, 2021 19

Non-Polar Bond �Joining of atoms with the same electronegativity Friday, December 31, 2021 20

Further Insight Into Electronegativity & Chemical Bonds Friday, December 31, 2021 21

Introduction �Electrons, of atoms such as oxygen and nitrogen, surrounding the nucleus of an atom, do not evenly distribute themselves evenly but tend to collect in one position. �As a result, the region where electrons are “collected” will be more negative than the rest of the atom. � Atoms with this uneven distribution of charge is said to be polarized. � This allows atoms to be considered electronegative. 12/31/2021 4: 27: 13 AM 22
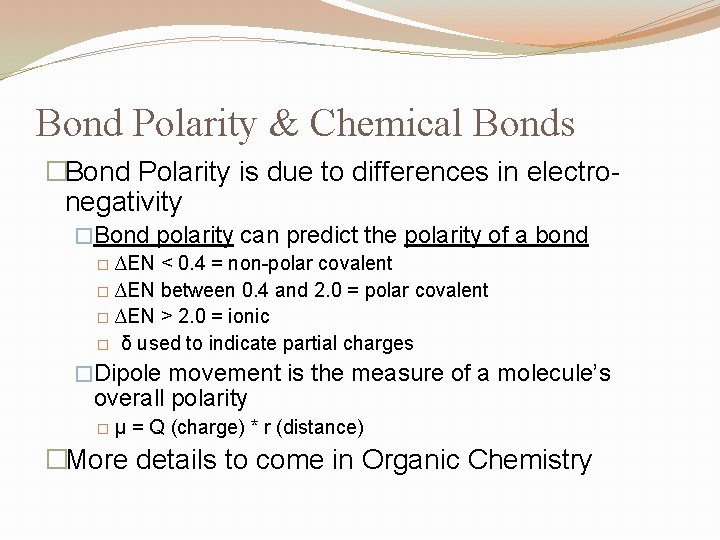
Bond Polarity & Chemical Bonds �Bond Polarity is due to differences in electronegativity �Bond polarity can predict the polarity of a bond � ∆EN < 0. 4 = non-polar covalent � ∆EN between 0. 4 and 2. 0 = polar covalent � ∆EN > 2. 0 = ionic � δ used to indicate partial charges �Dipole movement is the measure of a molecule’s overall polarity � μ = Q (charge) * r (distance) �More details to come in Organic Chemistry
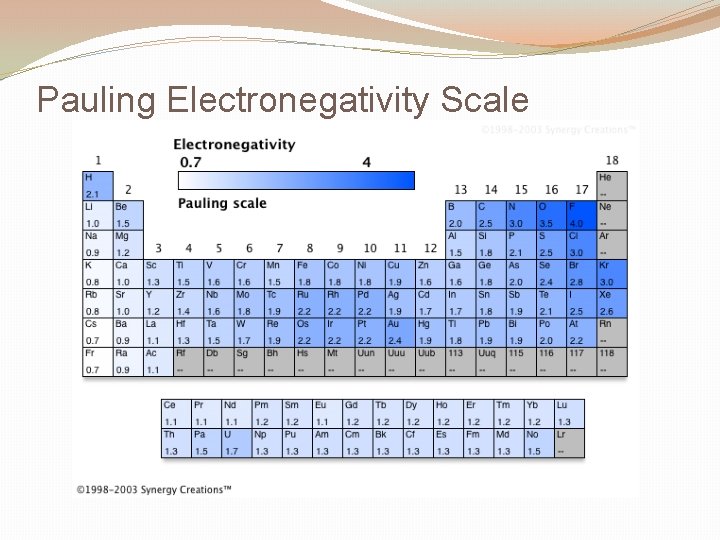
Pauling Electronegativity Scale

Types of Chemical Bonds Hydrogen Bonds Friday, December 31, 2021 25
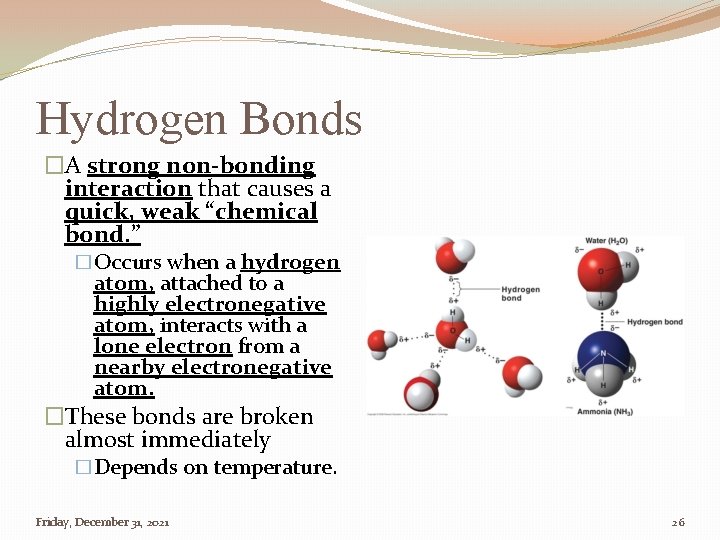
Hydrogen Bonds �A strong non-bonding interaction that causes a quick, weak “chemical bond. ” �Occurs when a hydrogen atom, attached to a highly electronegative atom, interacts with a lone electron from a nearby electronegative atom. �These bonds are broken almost immediately �Depends on temperature. Friday, December 31, 2021 26
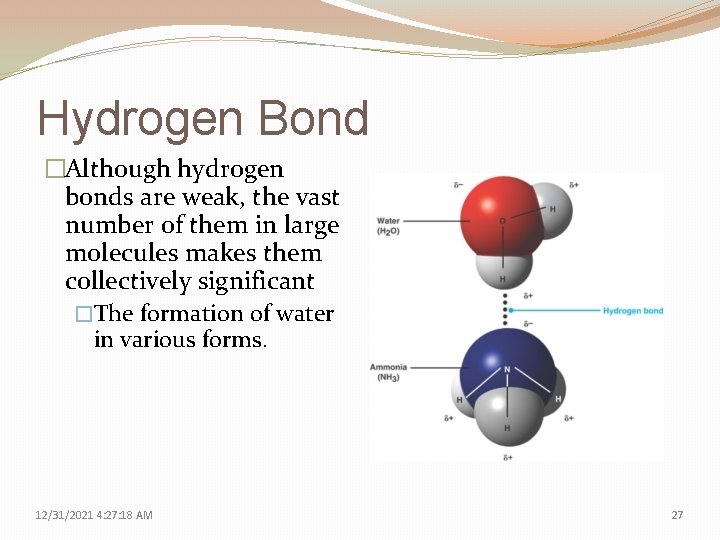
Hydrogen Bond �Although hydrogen bonds are weak, the vast number of them in large molecules makes them collectively significant �The formation of water in various forms. 12/31/2021 4: 27: 18 AM 27

Phases of Matter �Liquid �Solid �Gas �Elements and molecules can exist in all three phases Friday, December 31, 2021 28
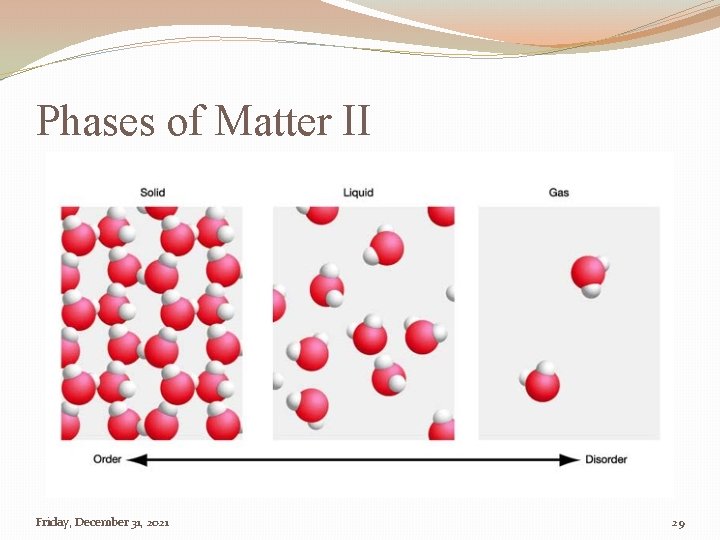
Phases of Matter II Friday, December 31, 2021 29
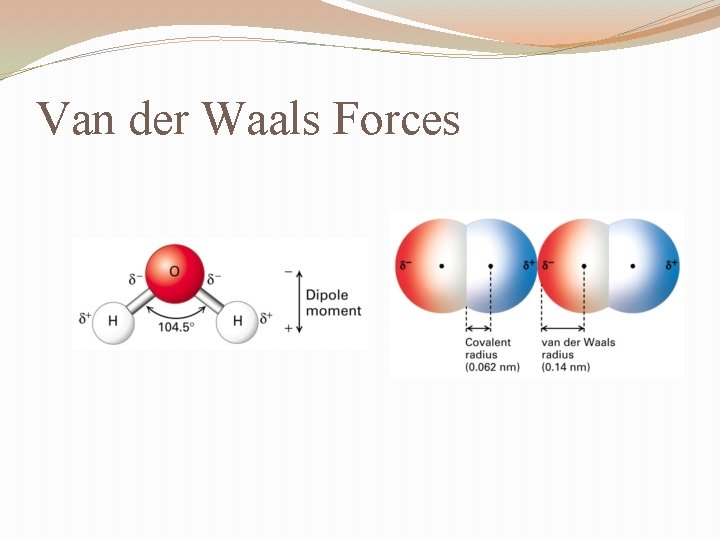
Van der Waals Forces

Isomers Friday, December 31, 2021 31

Isomers �Isomer �One of two, or more compounds, with the same chemical formula but different structural formula Friday, December 31, 2021 32
Isomers II �Stereoisomer �Atoms are connected in the same order but have different 3 D arrangements �Enantiomer � Stereoisomer that have a mirror-image relationship �Diastereoisomer � Stereoisomer that is not a mirror image. Friday, December 31, 2021 33
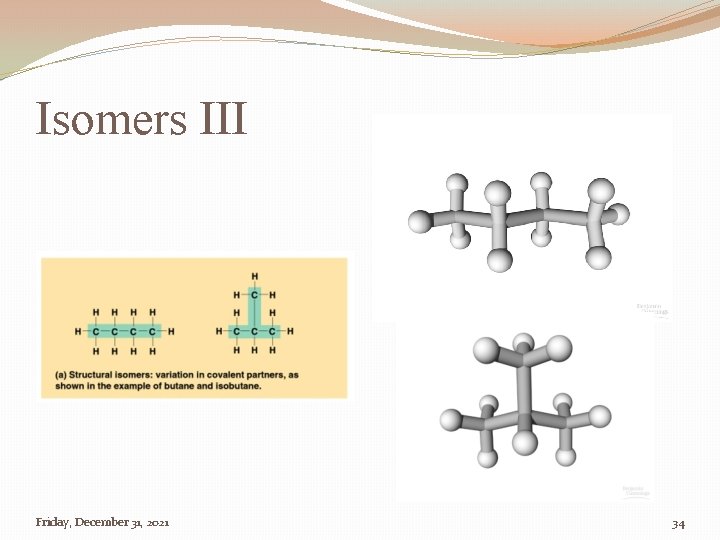
Isomers III Friday, December 31, 2021 34
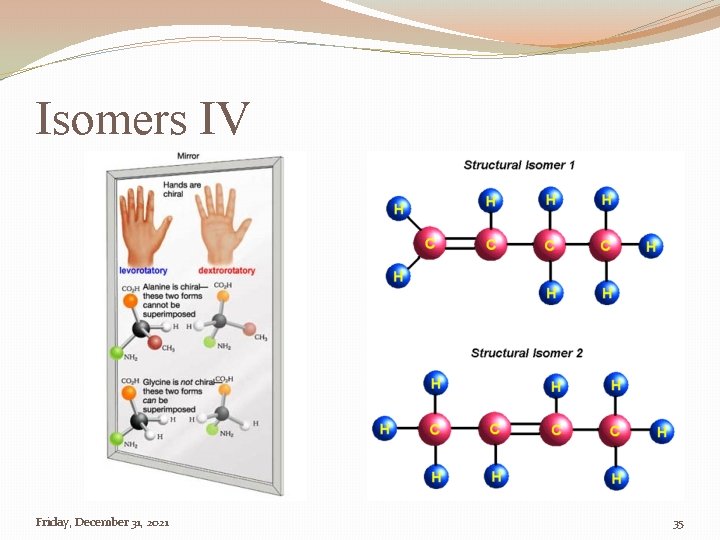
Isomers IV Friday, December 31, 2021 35
Importance of Recognizing Isomers �Sugars �There are different isomers of sugars used in different steps during cell respiration and photosynthesis. � Macromolecule Packets �Hormones & Steroids �Different isomers of hormones, and steroids, are critical to proper development and sexual development. 12/31/2021 4: 27: 24 AM 36
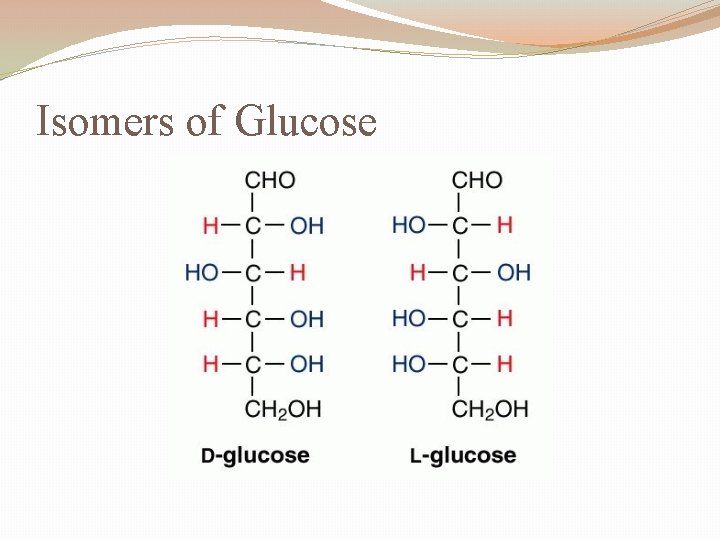
Isomers of Glucose
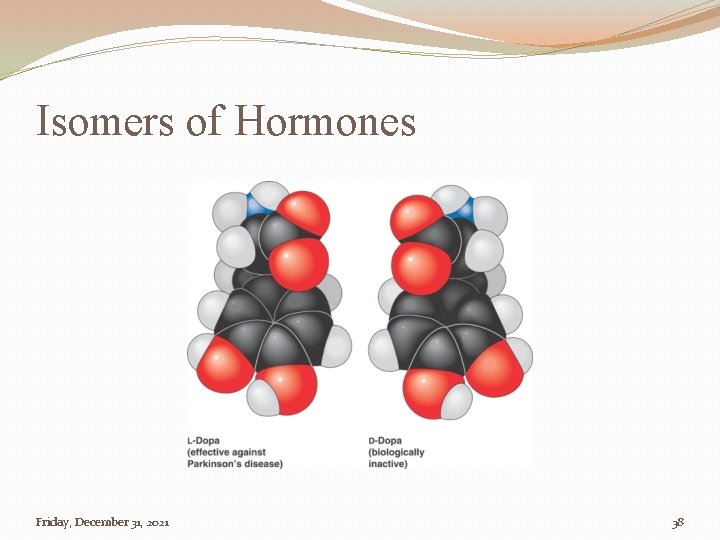
Isomers of Hormones Friday, December 31, 2021 38
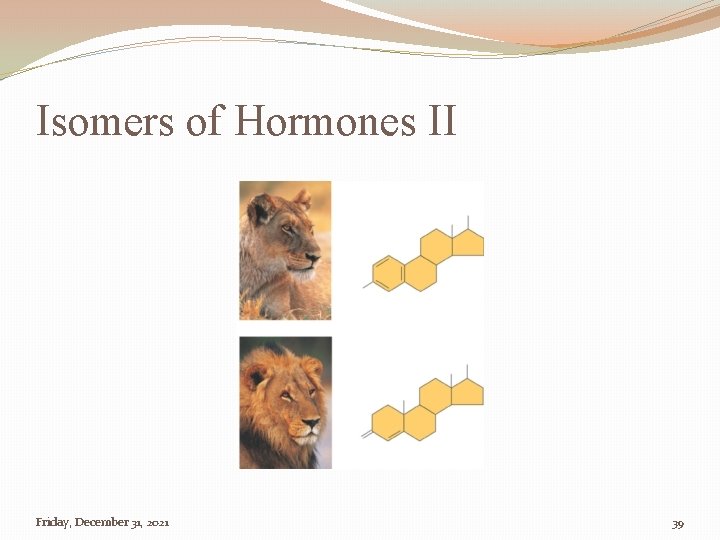
Isomers of Hormones II Friday, December 31, 2021 39
- Slides: 39