Molecules and Compounds Nomenclature Molecular compound Ionic compound




















- Slides: 59

Molecules and Compounds: Nomenclature: Molecular compound Ionic compound Acid

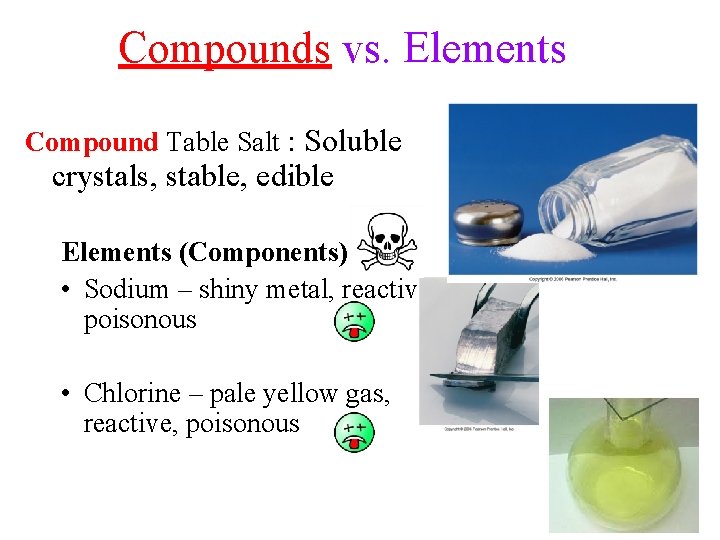
Compounds vs. Elements Compound Table Salt : Soluble crystals, stable, edible Elements (Components) • Sodium – shiny metal, reactive, poisonous • Chlorine – pale yellow gas, reactive, poisonous 2

Law of Constant Composition Pure substances have constant composition ü all samples of a pure substance contain the same elements in the same percentages (ratios): Water (H: 11%, O: 89%), Table salt (Na: 39%, Cl: 61%), Sugar Mixtures have variable composition: Air, Seawater, Concrete, Rocky road ice cream, Coke 3

Chemical Formula Chemical formula shows the number and type of each atom in the simplest unit of the compound • Element as letter symbol: H (for hydrogen); Na (for Sodium) • #Atoms of each element = subscript on the lower right of Symbol, H 2 O (two H atoms, one O atom, the 1 subscript is not written) • Polyatomic groups (multiple atoms in group, example: SO 4) are placed in parentheses if more than one. Example: Al 2(SO 4)3 (two Al atom, three SO 4 4 groups)

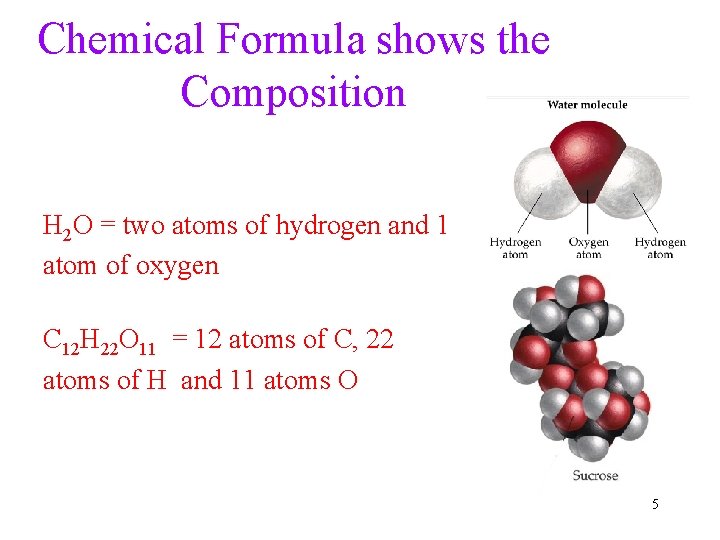
Chemical Formula shows the Composition H 2 O = two atoms of hydrogen and 1 atom of oxygen C 12 H 22 O 11 = 12 atoms of C, 22 atoms of H and 11 atoms O 5

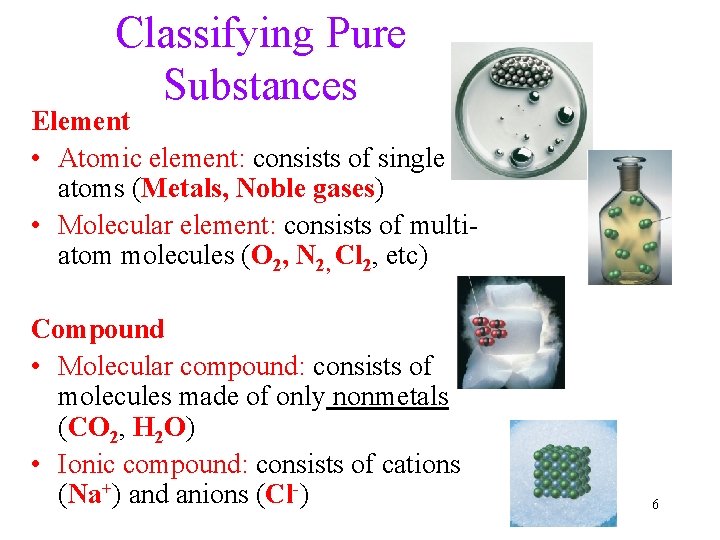
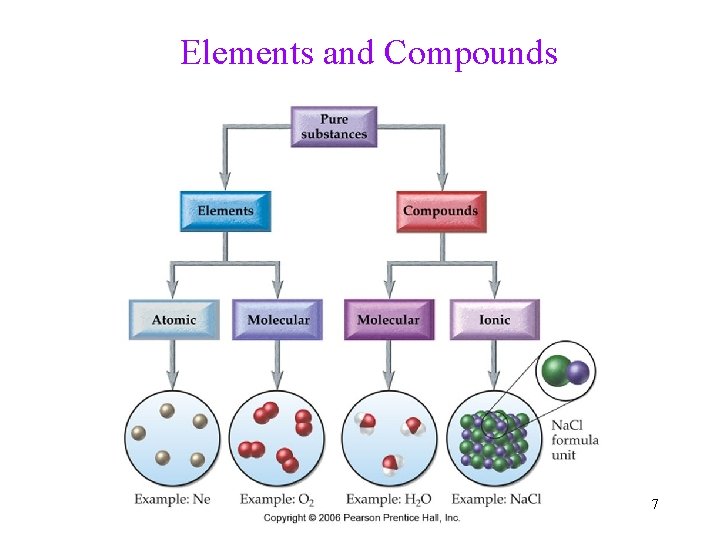
Classifying Pure Substances Element • Atomic element: consists of single atoms (Metals, Noble gases) • Molecular element: consists of multiatom molecules (O 2, N 2, Cl 2, etc) Compound • Molecular compound: consists of molecules made of only nonmetals (CO 2, H 2 O) • Ionic compound: consists of cations (Na+) and anions (Cl-) 6

Elements and Compounds 7

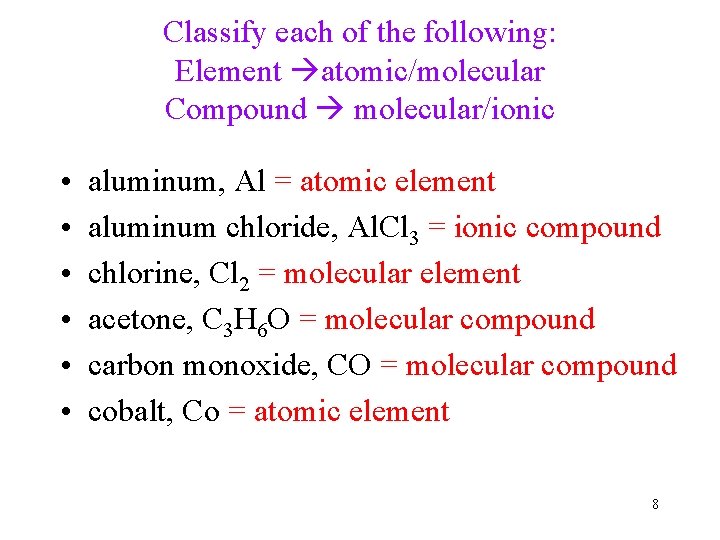
Classify each of the following: Element atomic/molecular Compound molecular/ionic • • • aluminum, Al = atomic element aluminum chloride, Al. Cl 3 = ionic compound chlorine, Cl 2 = molecular element acetone, C 3 H 6 O = molecular compound carbon monoxide, CO = molecular compound cobalt, Co = atomic element 8

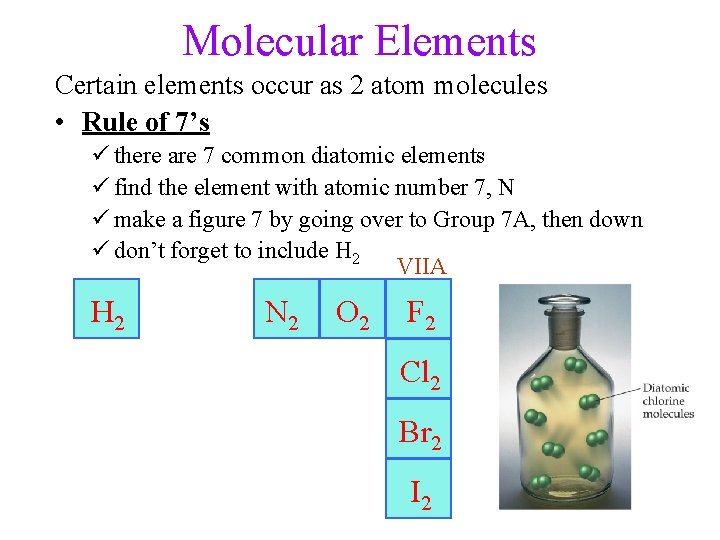
Molecular Elements Certain elements occur as 2 atom molecules • Rule of 7’s ü there are 7 common diatomic elements ü find the element with atomic number 7, N ü make a figure 7 by going over to Group 7 A, then down ü don’t forget to include H 2 VIIA H 2 N 2 O 2 F 2 Cl 2 Br 2 I 2 9

Molecular Elements = Metalloid H = Nonmetal N O F Cl Br I

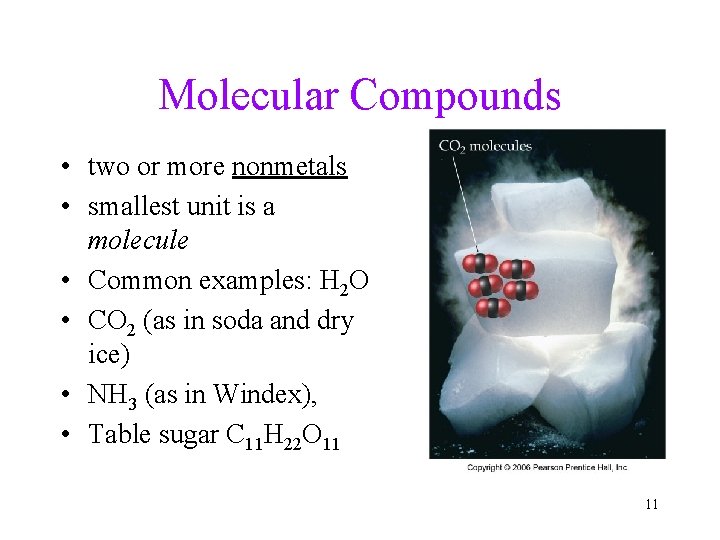
Molecular Compounds • two or more nonmetals • smallest unit is a molecule • Common examples: H 2 O • CO 2 (as in soda and dry ice) • NH 3 (as in Windex), • Table sugar C 11 H 22 O 11 11

Ionic Compounds Ions: Metals (Cation Mx+) and Nonmetals (Anion Ny -) • No individual molecules!! • have a 3 -dimensional array of cations and anions made of formula units: Na. Cl, Mg. O • Na+ Cl- Na+ Cl • Cl- Na+ • Na+ Cl- 12

Naming Binary Molecular Compounds 1. Name first element in formula first ü use the full name of the element 2. Name the second element in the formula with an -ide ü as if it were an anion, however, remember these compounds do not contain ions! 3. Use a prefix in front of each name to indicate the number of atoms a) Never use the prefix mono- on the first element 13

Subscript - Prefixes • 1 = mono-; ünot used on first nonmetal • 2 = di • 3 = tri • 4 = tetra- • • • 5 = penta 6 = hexa 7 = hepta 8 = octadrop last “a” if name begins with vowel 14

Exceptions when Naming Molecular Compounds of course, water Other common exceptions: • NH 3: ammonia (as in Windex) • H 2 S: hydrogen sulfide • HCl: hydrogen chloride (same for HX, where X = halogen) • CH 4: methane (as in natural gas) • H 2 O 2: hydrogen peroxide 15

Example – Naming N 2 O 4 and BF 3 1. Is it one of the common exceptions? (H 2 O, NH 3, CH 4, C 12 H 22 O 11 2. Identify Major Class (Molecular or Ionic) 3. Name the first element 4. Name the second element with an –ide 5. Add a prefix to each name to indicate the subscript: Write the first element with prefix, then the second element with prefix ü Drop prefix mono from first element 16

Practice: Naming Molecular Compounds • SF 4 • • • Mono Di Tri Tetra Penta Hexa Hepta Octa Nona Deca • I 2 O 7 17

Ionic Compounds • Made of Cation (+) and Anion (-) • Name: Cation Anion example: Na. Cl Sodium Chloride üCation: ØType I metal ØType II metal ØPolyatomic ion: ammonium NH 4+ üAnion: ØNonmetal: Chloride Cl-, Oxide O 2ØPolyatomic ion: SO 42 - , OH- , NO 318

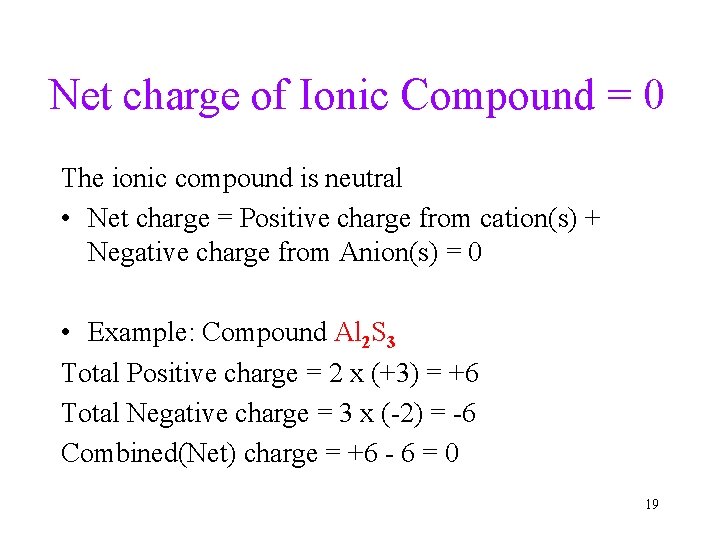
Net charge of Ionic Compound = 0 The ionic compound is neutral • Net charge = Positive charge from cation(s) + Negative charge from Anion(s) = 0 • Example: Compound Al 2 S 3 Total Positive charge = 2 x (+3) = +6 Total Negative charge = 3 x (-2) = -6 Combined(Net) charge = +6 - 6 = 0 19

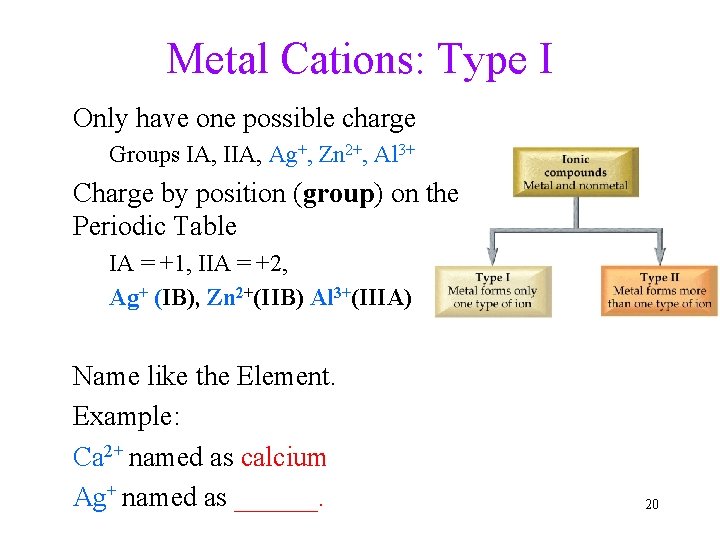
Metal Cations: Type I Only have one possible charge Groups IA, IIA, Ag+, Zn 2+, Al 3+ Charge by position (group) on the Periodic Table IA = +1, IIA = +2, Ag+ (IB), Zn 2+(IIB) Al 3+(IIIA) Name like the Element. Example: Ca 2+ named as calcium Ag+ named as ______. 20

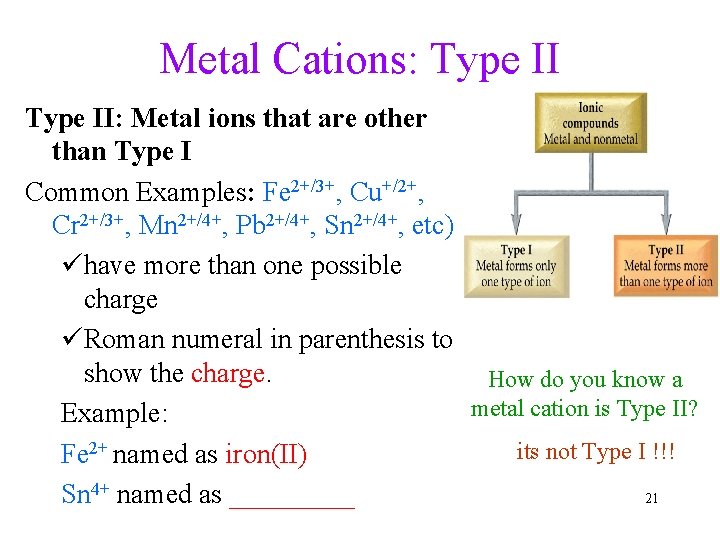
Metal Cations: Type II: Metal ions that are other than Type I Common Examples: Fe 2+/3+, Cu+/2+, Cr 2+/3+, Mn 2+/4+, Pb 2+/4+, Sn 2+/4+, etc) ühave more than one possible charge üRoman numeral in parenthesis to show the charge. How do you know a metal cation is Type II? Example: its not Type I !!! Fe 2+ named as iron(II) Sn 4+ named as _____ 21

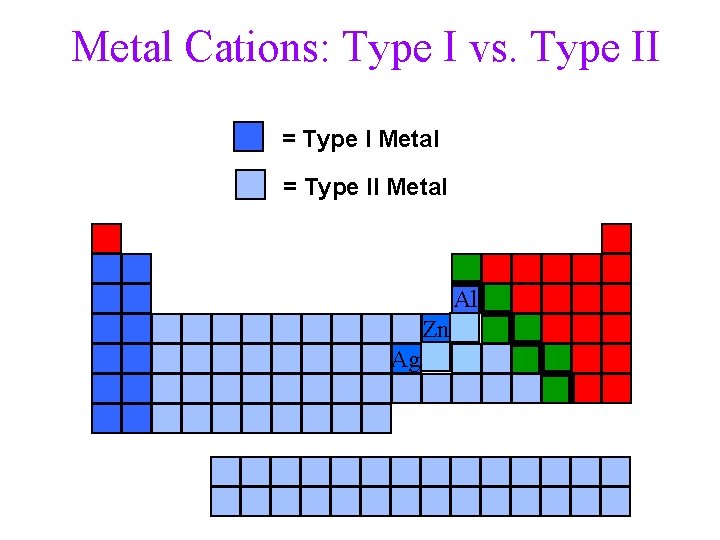
Metal Cations: Type I vs. Type II = Type I Metal = Type II Metal Al Zn Ag

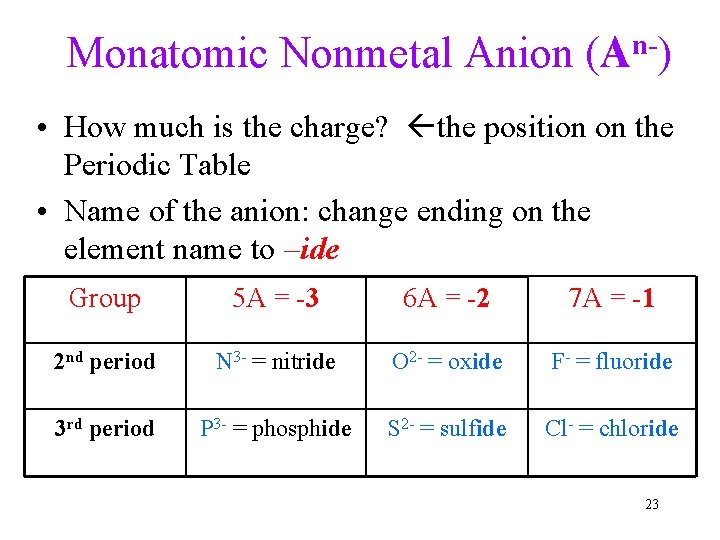
Monatomic Nonmetal Anion (An-) • How much is the charge? the position on the Periodic Table • Name of the anion: change ending on the element name to –ide Group 5 A = -3 6 A = -2 7 A = -1 2 nd period N 3 - = nitride O 2 - = oxide F- = fluoride 3 rd period P 3 - = phosphide S 2 - = sulfide Cl- = chloride 23

Name of Ionic Compounds • Name: Cation Anion: Sodium Chloride üCation: ØType I metal = metal name: Na+ => Sodium, Mg 2+ => Magnesium ØType II metal = metal name(charge): Fe 3+ => Iron(III), Cu 2+ Copper(II) ØPolyatomic ion = name of polyatomic ion, NH 4+ => Ammonium üAnion: ØNonmetal = stem of nonmetal name + ide, Chloride, Oxide 24

Example – Name Ca. F 2, Al 2 O 3 1. Is it one of the common exceptions? H 2 O, NH 3, CH 4, C 12 H 22 O 11 2. Identify Major Class (Ionic or Molecular) 3. Identify cation and anion 4. Is the metal Type I or Type II (Type I = IA, IIA, AZA)? If type I, remember the charge. 5. Name the cation first, then anion if type I, cation is simply the name of the element. 25

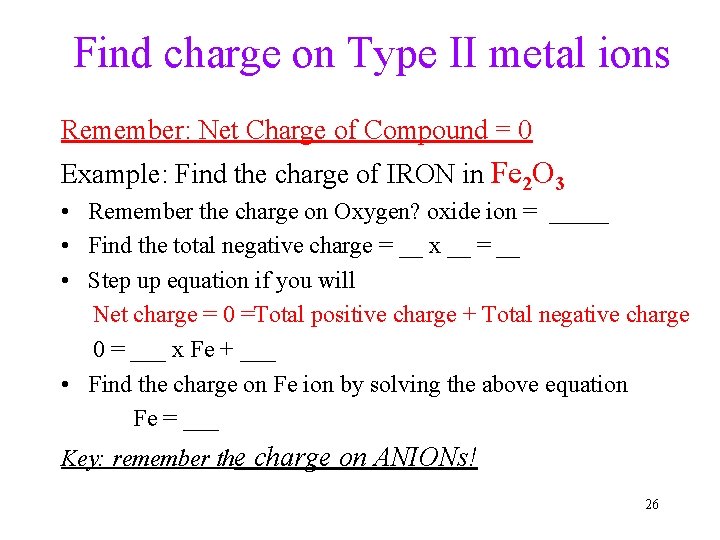
Find charge on Type II metal ions Remember: Net Charge of Compound = 0 Example: Find the charge of IRON in Fe 2 O 3 • Remember the charge on Oxygen? oxide ion = _____ • Find the total negative charge = __ x __ = __ • Step up equation if you will Net charge = 0 =Total positive charge + Total negative charge 0 = ___ x Fe + ___ • Find the charge on Fe ion by solving the above equation Fe = ___ Key: remember the charge on ANIONs! 26

Example – Naming Cu 2 S, Sn. F 4 1. Is it one of the common exceptions? H 2 O, NH 3, CH 4, C 12 H 22 O 11 2. Identify Major Class (Ionic or Molecular) 3. Identify cation and anion 4. Is the metal Type I or Type II (Type I = IA, IIA, AZA)? 5. If Type II metal ion, find the charge on cation 6. Name the cation first, then anion. If type II metal ion, name(Roman numeral) 27

Practice: Naming Ionic compounds • Hg 2 F 2 • Fe 2 S 3 • Ag 2 S 28

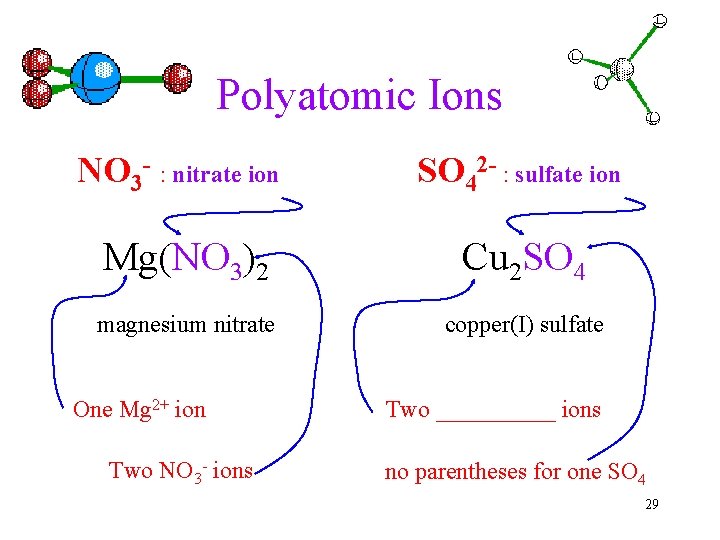
Polyatomic Ions NO 3 - : nitrate ion SO 42 - : sulfate ion Mg(NO 3)2 Cu 2 SO 4 magnesium nitrate copper(I) sulfate One Mg 2+ ion Two NO 3 - ions Two _____ ions no parentheses for one SO 4 29

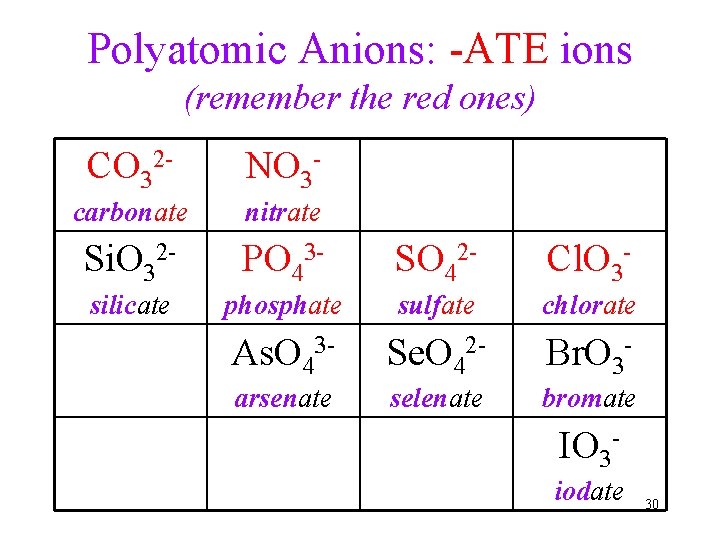
Polyatomic Anions: -ATE ions (remember the red ones) CO 32 - NO 3 - carbonate nitrate Si. O 32 - PO 43 - SO 42 - Cl. O 3 - silicate phosphate sulfate chlorate As. O 43 - Se. O 42 - Br. O 3 - arsenate selenate bromate IO 3 iodate 30

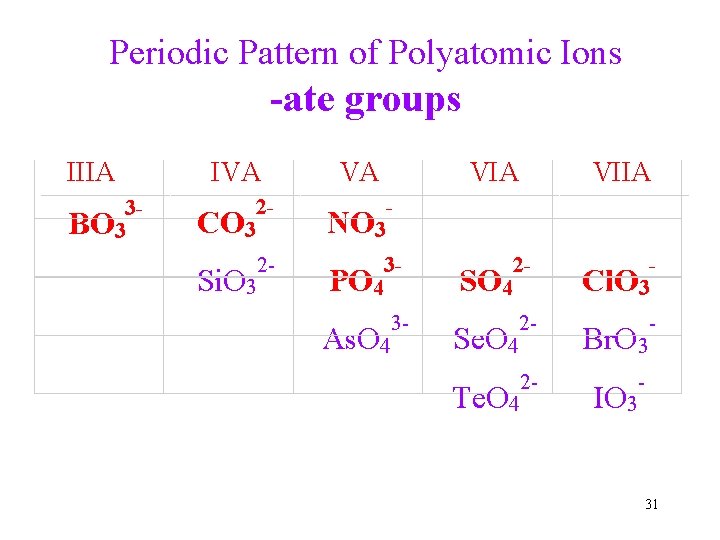
Periodic Pattern of Polyatomic Ions -ate groups IIIA 3 BO 3 IVA VA VIIA 2 CO 3 NO 3 2 Si. O 3 3 PO 4 2 SO 4 Cl. O 3 3 As. O 4 2 Se. O 4 Br. O 3 2 Te. O 4 IO 3 31

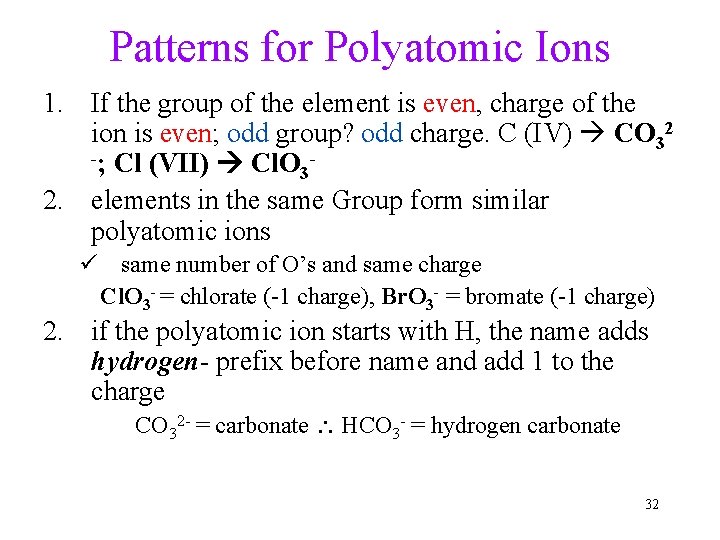
Patterns for Polyatomic Ions 1. If the group of the element is even, charge of the ion is even; odd group? odd charge. C (IV) CO 32 -; Cl (VII) Cl. O 3 2. elements in the same Group form similar polyatomic ions ü same number of O’s and same charge Cl. O 3 - = chlorate (-1 charge), Br. O 3 - = bromate (-1 charge) 2. if the polyatomic ion starts with H, the name adds hydrogen- prefix before name and add 1 to the charge CO 32 - = carbonate HCO 3 - = hydrogen carbonate 32

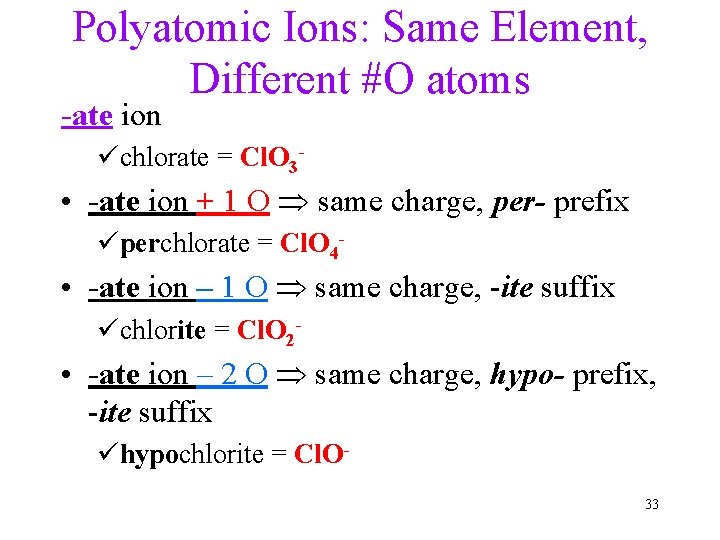
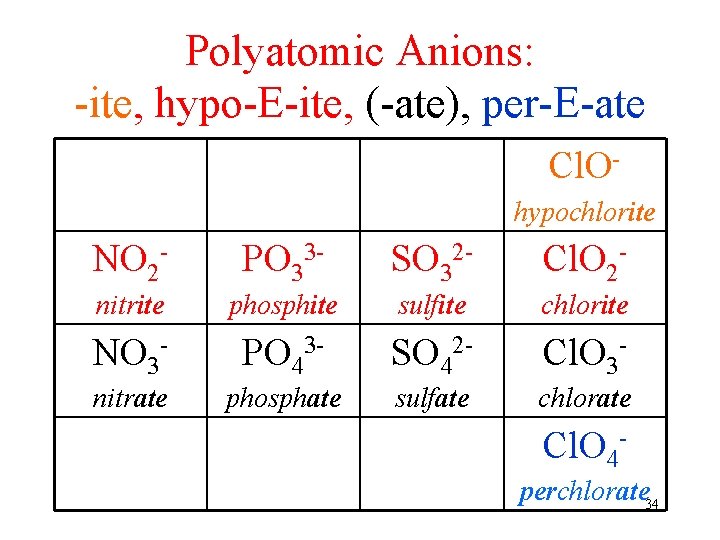
Polyatomic Ions: Same Element, Different #O atoms -ate ion üchlorate = Cl. O 3 - • -ate ion + 1 O same charge, per- prefix üperchlorate = Cl. O 4 - • -ate ion – 1 O same charge, -ite suffix üchlorite = Cl. O 2 - • -ate ion – 2 O same charge, hypo- prefix, -ite suffix ühypochlorite = Cl. O 33

Polyatomic Anions: -ite, hypo-E-ite, (-ate), per-E-ate Cl. Ohypochlorite NO 2 - PO 33 - SO 32 - Cl. O 2 - nitrite phosphite sulfite chlorite NO 3 - PO 43 - SO 42 - Cl. O 3 - nitrate phosphate sulfate chlorate Cl. O 4 perchlorate 34

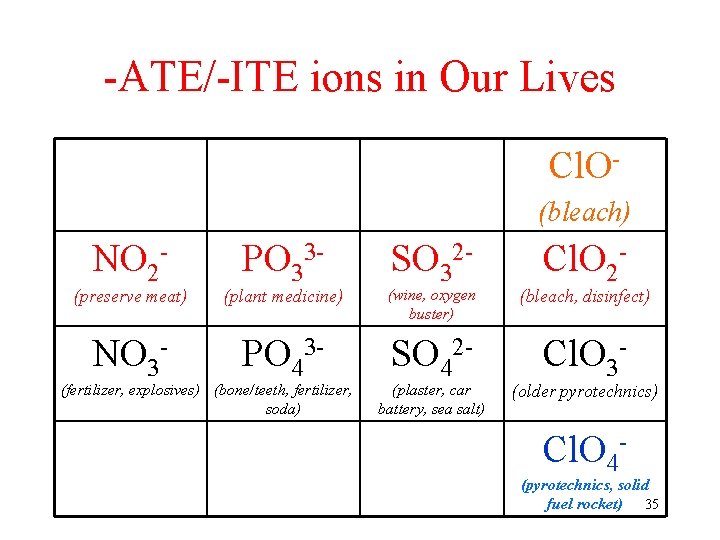
-ATE/-ITE ions in Our Lives Cl. O(bleach) NO 2 - PO 33 - SO 32 - Cl. O 2 - (preserve meat) (plant medicine) (wine, oxygen buster) (bleach, disinfect) NO 3 - PO 43 - SO 42 - Cl. O 3 - (fertilizer, explosives) (bone/teeth, fertilizer, soda) (plaster, car battery, sea salt) (older pyrotechnics) Cl. O 4 - (pyrotechnics, solid fuel rocket) 35

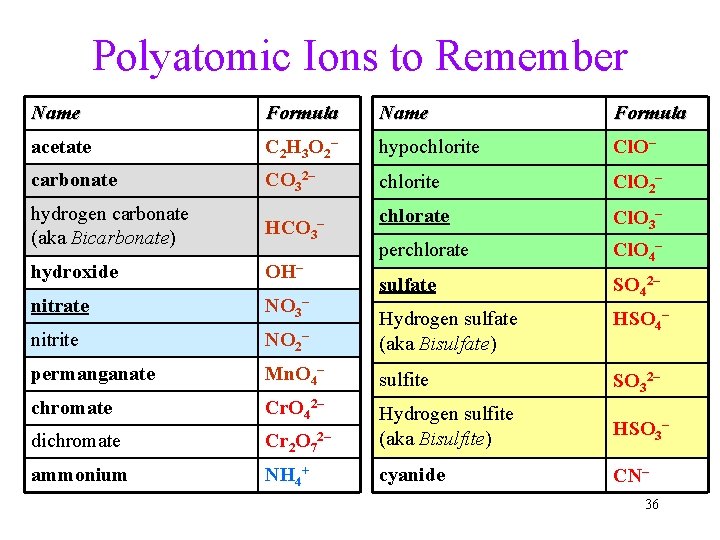
Polyatomic Ions to Remember Name Formula acetate C 2 H 3 O 2 – hypochlorite Cl. O– carbonate CO 32– chlorite Cl. O 2– hydrogen carbonate (aka Bicarbonate) HCO 3 chlorate Cl. O 3– hydroxide OH– perchlorate Cl. O 4– nitrate NO 3– sulfate SO 42– nitrite NO 2– Hydrogen sulfate (aka Bisulfate) HSO 4– permanganate Mn. O 4– sulfite SO 32– chromate Cr. O 42– dichromate Cr 2 O 7 Hydrogen sulfite (aka Bisulfite) HSO 3– ammonium NH 4+ cyanide CN– – 2– 36

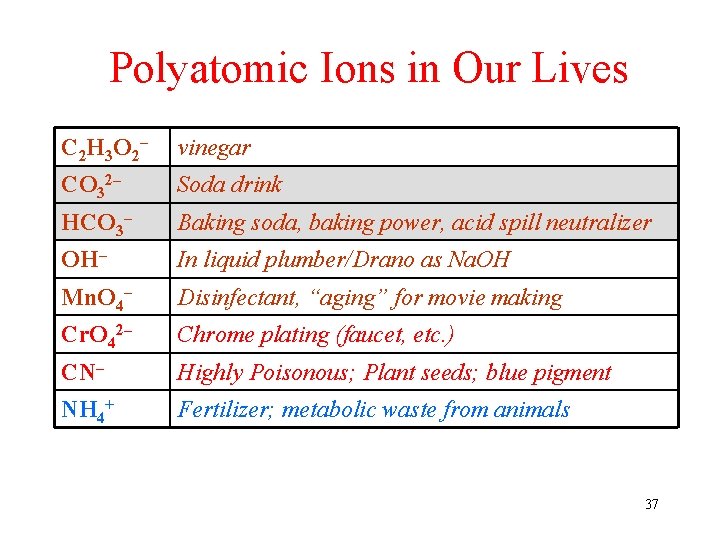
Polyatomic Ions in Our Lives C 2 H 3 O 2– vinegar CO 32– Soda drink HCO 3– Baking soda, baking power, acid spill neutralizer OH– In liquid plumber/Drano as Na. OH Mn. O 4– Disinfectant, “aging” for movie making Cr. O 42– Chrome plating (faucet, etc. ) CN– Highly Poisonous; Plant seeds; blue pigment NH 4+ Fertilizer; metabolic waste from animals 37

Example – Naming 1. 2. 3. 4. 5. Fe(NO 3)3 , (NH 4)3 PO 3 One of the common exceptions? H 2 O, NH 3, CH 4, C 12 H 22 O 11 Identify Major Class (ionic or molecular) If metal as cation, Type I or Type II? If type II, find the charge of metal ion (starting from charge of anion in your memory) Name cation first, followed by anion ______ nitrate, ____________ 38

Practice: Naming Ionic compounds • Hg 2 SO 4 • Fe. CO 3 • Co. PO 4 • Zn(NO 3)2 39

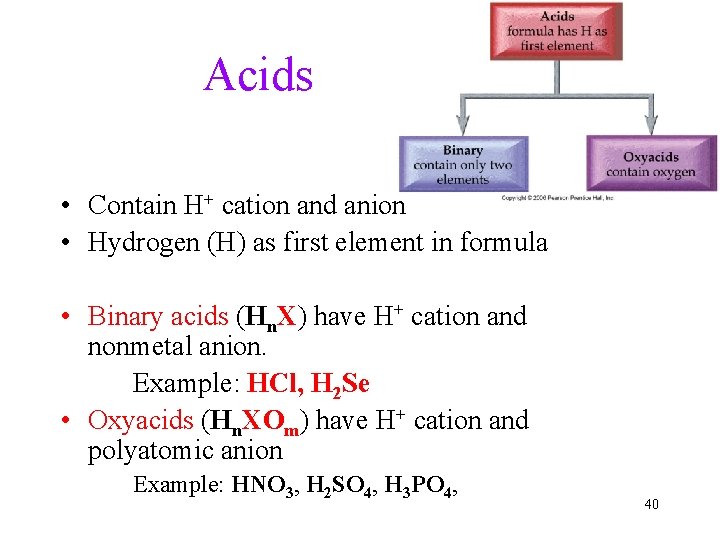
Acids • Contain H+ cation and anion • Hydrogen (H) as first element in formula • Binary acids (Hn. X) have H+ cation and nonmetal anion. Example: HCl, H 2 Se • Oxyacids (Hn. XOm) have H+ cation and polyatomic anion Example: HNO 3, H 2 SO 4, H 3 PO 4, 40

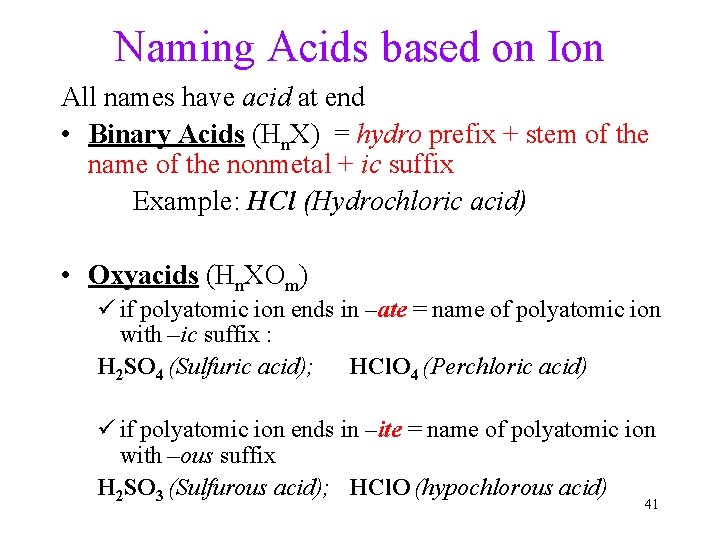
Naming Acids based on Ion All names have acid at end • Binary Acids (Hn. X) = hydro prefix + stem of the name of the nonmetal + ic suffix Example: HCl (Hydrochloric acid) • Oxyacids (Hn. XOm) ü if polyatomic ion ends in –ate = name of polyatomic ion with –ic suffix : H 2 SO 4 (Sulfuric acid); HCl. O 4 (Perchloric acid) ü if polyatomic ion ends in –ite = name of polyatomic ion with –ous suffix H 2 SO 3 (Sulfurous acid); HCl. O (hypochlorous acid) 41

Naming Acids: 1. 2. HF 3. HCl. O 3 4. HNO 2 Identify if the compound is an acid Identify the anion ( -ide, -ite, or –ate? ) Rename the anion with prefix and/or suffix: ü -ide hydro-xxx -ic ü -ite -ous ü -ate -ic Add the word acid to the end 42

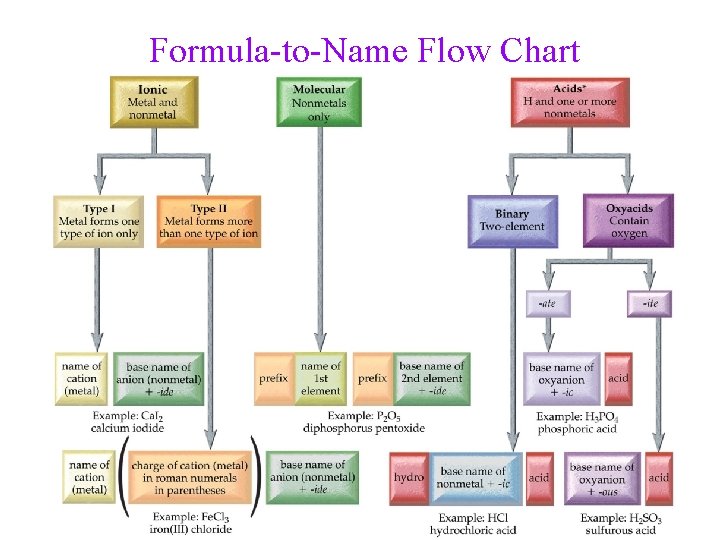
Formula-to-Name Flow Chart 43

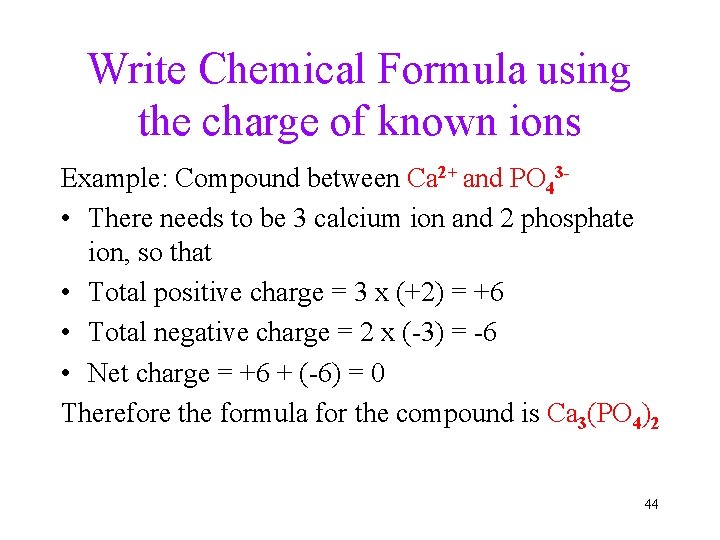
Write Chemical Formula using the charge of known ions Example: Compound between Ca 2+ and PO 43 • There needs to be 3 calcium ion and 2 phosphate ion, so that • Total positive charge = 3 x (+2) = +6 • Total negative charge = 2 x (-3) = -6 • Net charge = +6 + (-6) = 0 Therefore the formula for the compound is Ca 3(PO 4)2 44

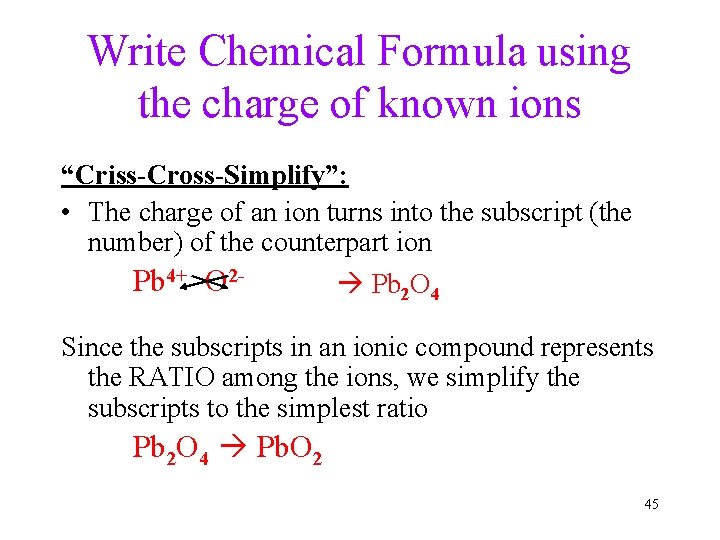
Write Chemical Formula using the charge of known ions “Criss-Cross-Simplify”: • The charge of an ion turns into the subscript (the number) of the counterpart ion Pb 4+ O 2 - Pb 2 O 4 Since the subscripts in an ionic compound represents the RATIO among the ions, we simplify the subscripts to the simplest ratio Pb 2 O 4 Pb. O 2 45

Write formula sodium phosphate chromium(III) selenide iodic acid Na 3 PO 4, 1. Identify if the compound is an acid 2. Identify cation and anion: find the charge Type II metal ion is given as the Roman numeral 3. “Criss-Cross. Simplify” Cr 2 Se 3 HIO 3 46

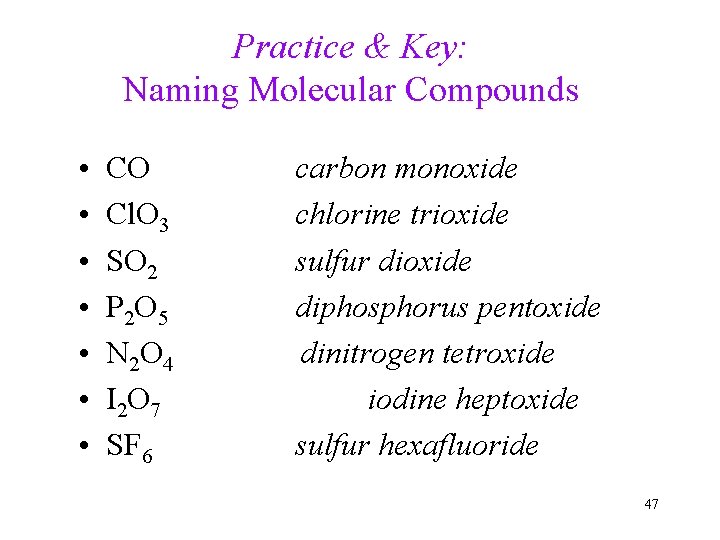
Practice & Key: Naming Molecular Compounds • • CO Cl. O 3 SO 2 P 2 O 5 N 2 O 4 I 2 O 7 SF 6 carbon monoxide chlorine trioxide sulfur dioxide diphosphorus pentoxide dinitrogen tetroxide iodine heptoxide sulfur hexafluoride 47

Answer key: names of ionic compounds • • Hg. F 2 = Mercury(II) fluoride Cu. I 2 = copper(II) iodide Ca. Cl 2 = calcium chloride Fe 2 S 3 = Iron(III) sulfide Sn. Br 4 = tin(IV) bromide Mg 3 N 2 = magnesium nitride Ag 2 S = silver sulfide 48

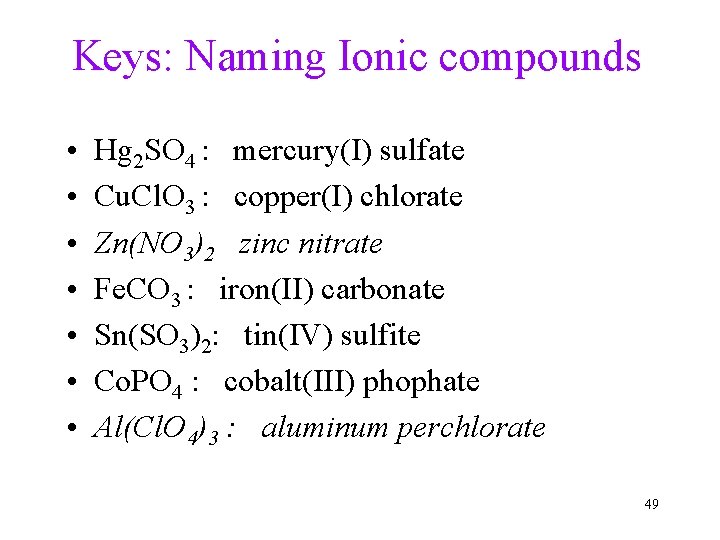
Keys: Naming Ionic compounds • • Hg 2 SO 4 : mercury(I) sulfate Cu. Cl. O 3 : copper(I) chlorate Zn(NO 3)2 zinc nitrate Fe. CO 3 : iron(II) carbonate Sn(SO 3)2: tin(IV) sulfite Co. PO 4 : cobalt(III) phophate Al(Cl. O 4)3 : aluminum perchlorate 49

Practice: Writing formulas (I) • • copper(II) chloride aluminum oxide magnesium phosphide iron(II) bromide lead(II) sulfide zinc iodide sodium nitride 50

Key for Writing formulas (I): use criss-cross-reduce • • copper(II) chloride aluminum oxide magnesium phosphide iron(II) bromide lead(II) sulfide zinc iodide sodium nitride Cu. Cl 2 Al 2 O 3 Mg 3 P 2 Fe. Br 2 Pb. S Zn. I 2 Na 3 N 51

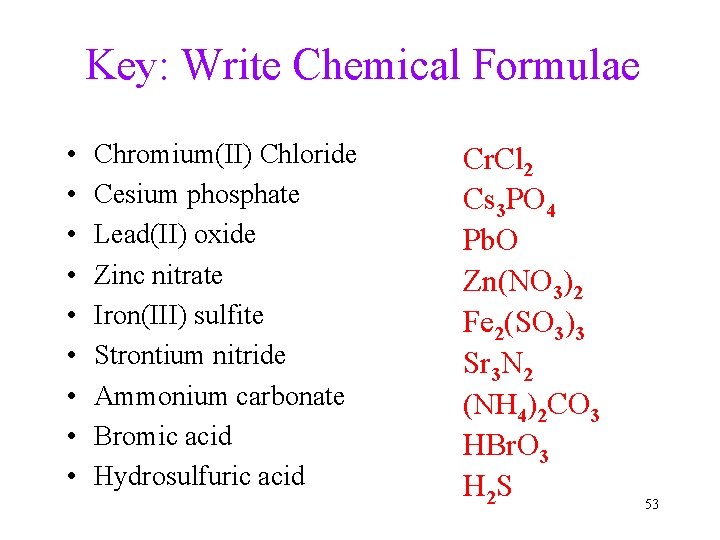
More Practice: Chemical Formulae • • • Chromium(II) Chloride Cesium phosphate Lead(II) oxide Zinc nitrate Iron(III) sulfite Strontium nitride Ammonium carbonate Bromic acid Hydrosulfuric acid 52

Key: Write Chemical Formulae • • • Chromium(II) Chloride Cesium phosphate Lead(II) oxide Zinc nitrate Iron(III) sulfite Strontium nitride Ammonium carbonate Bromic acid Hydrosulfuric acid Cr. Cl 2 Cs 3 PO 4 Pb. O Zn(NO 3)2 Fe 2(SO 3)3 Sr 3 N 2 (NH 4)2 CO 3 HBr. O 3 H 2 S 53

Review: Naming Compounds • • Cu. SO 3 Ag. Cl. O N 2 O 5 H 2 S Fe. I 2 Sn(NO 3)4 Ba 3(PO 4)2 (NH 4)2 S copper(II) sulfite silver hypochlorite dinitrogen pentoxide hydrosulfuric acid iron(II) iodide tin(IV) nitrate barium phosphate ammonium sulfide 54

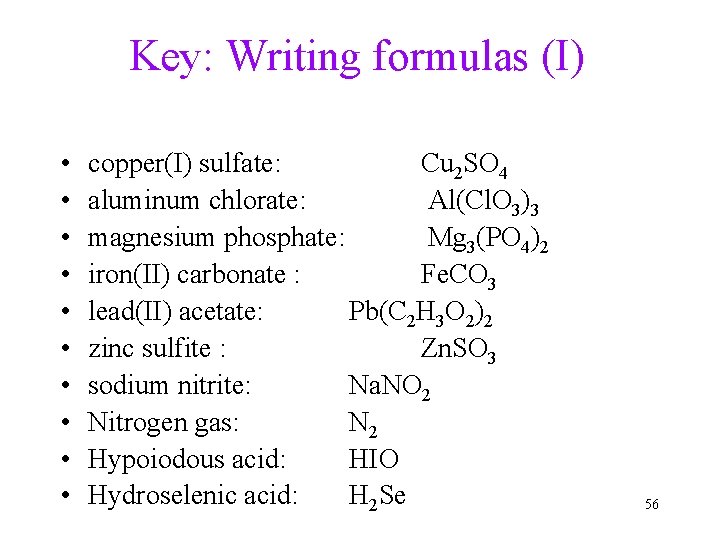
Practice: Writing formulas (I) • • • copper(I) sulfate aluminum chlorate magnesium phosphate iron(II) carbonate lead(II) acetate zinc sulfite sodium nitrite Nitrogen gas Hypoiodous acid Hydroselenic acid 55

Key: Writing formulas (I) • • • copper(I) sulfate: Cu 2 SO 4 aluminum chlorate: Al(Cl. O 3)3 magnesium phosphate: Mg 3(PO 4)2 iron(II) carbonate : Fe. CO 3 lead(II) acetate: Pb(C 2 H 3 O 2)2 zinc sulfite : Zn. SO 3 sodium nitrite: Na. NO 2 Nitrogen gas: N 2 Hypoiodous acid: HIO Hydroselenic acid: H 2 Se 56

More on Writing formulae • • copper(II) sulfate aluminum perchlorate hydroiodic acid iron(III) bromide Diphosphorus pentoxide lead(IV) nitride zinc carbonate helium gas 57

Key: Writing formulae • • • copper(II) sulfate Cu. SO 4 aluminum perchlorate Al(Cl. O 4)3 hydroiodic acid HI iron(III) bromide Fe. Br 3 Diphosphorus pentoxide P 2 O 5 lead(IV) nitride Pb 3 N 4 zinc carbonate Zn. CO 3 ammonium nitrite NH 4 NO 2 helium gas He 58

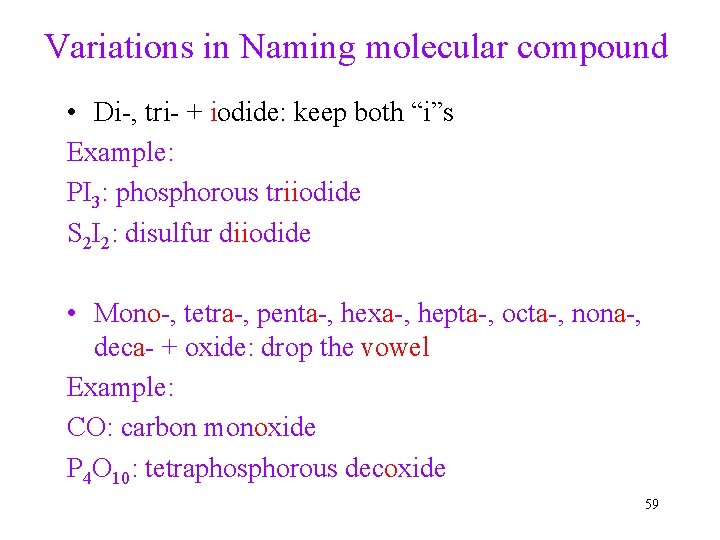
Variations in Naming molecular compound • Di-, tri- + iodide: keep both “i”s Example: PI 3: phosphorous triiodide S 2 I 2: disulfur diiodide • Mono-, tetra-, penta-, hexa-, hepta-, octa-, nona-, deca- + oxide: drop the vowel Example: CO: carbon monoxide P 4 O 10: tetraphosphorous decoxide 59