Molecular Workbenches Protein structure Life is a system
“Molecular Workbenches” Protein structure

Life is a system of elements that can replicate the entire set of elements from rudimentary parts to form new copies of the whole system.

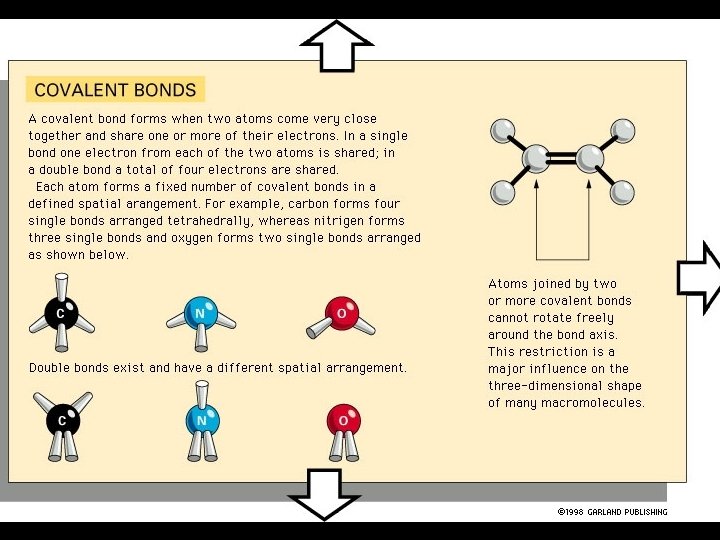
Elements: Are large, macromolecules, such as DNA, RNA and proteins, with 1000’s of covalently linked atoms. Rudimentary Parts: Are the building blocks for the elements. DNA and RNA are made from nucleic acids; proteins are made from amino acids. These are small molecules held together by covalent bonds.
Two Types of Atomic Interaction: Covalent Bonds (make molecules) Non-covalent Bonds (make molecules come alive)

Two Types of Covalent Bond: Non-polar (electronically balanced) Polar (electronically unbalanced)

Molecules “see” each other by non-covalent interactions of their electron shells.

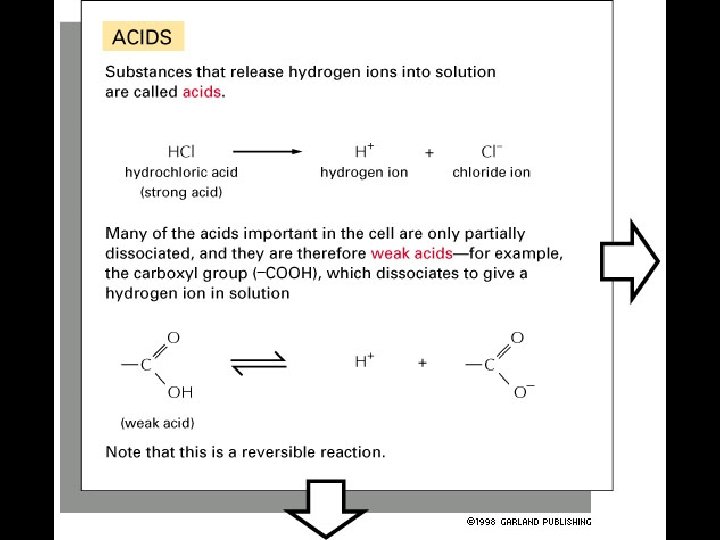
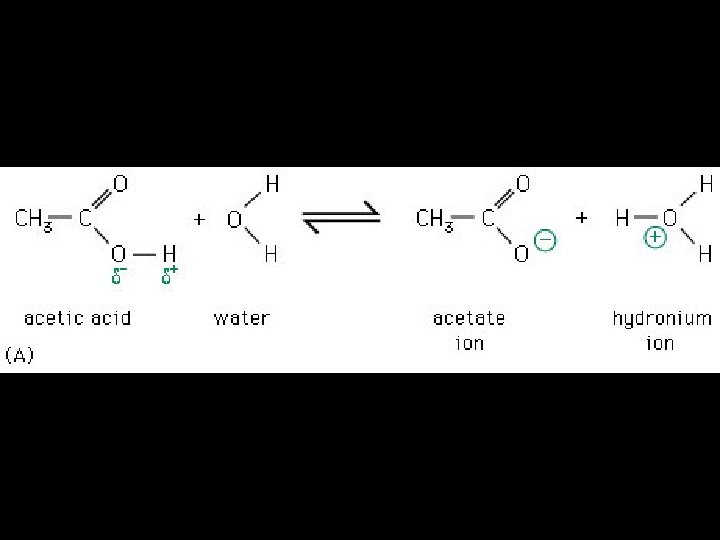
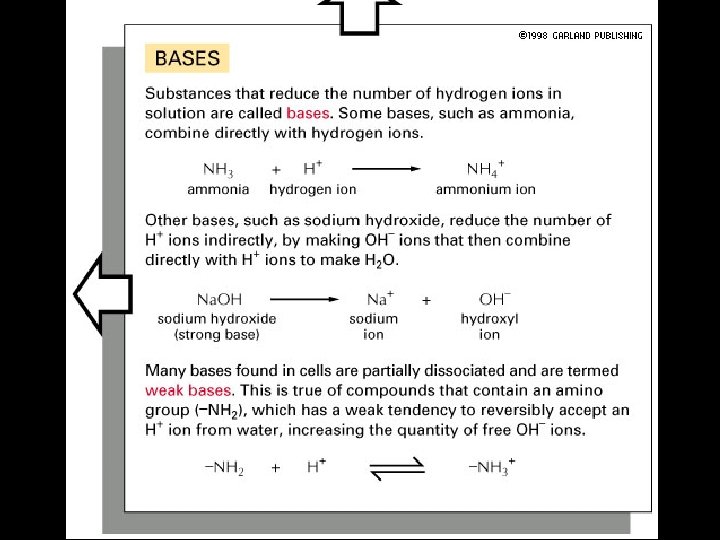
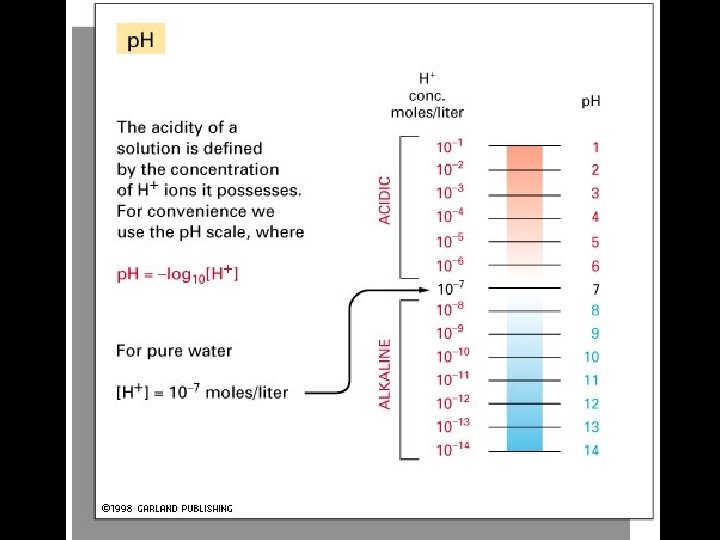
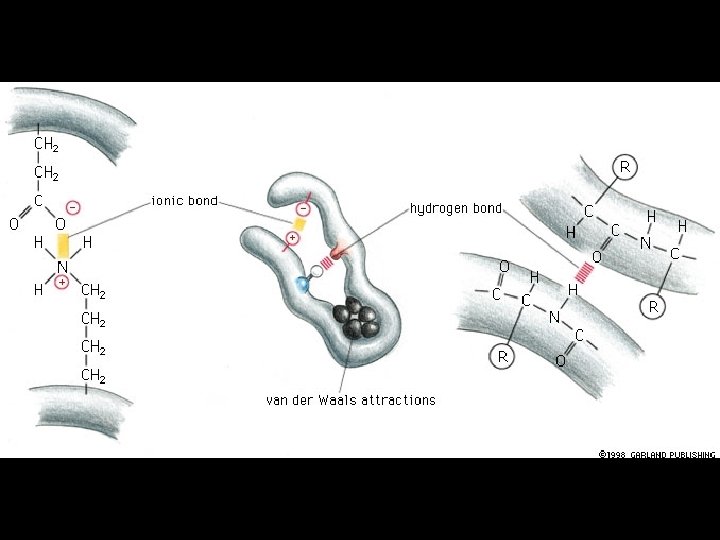
4 Types of Non-covalent Bonds: (1) van der Waals (2) hydrogen bonds (3) ionic (4) hydrophobic effect

Covalent and non-covalent Chemical bonds:

Living things have very high information content.

Proteins are amino acid polymers.

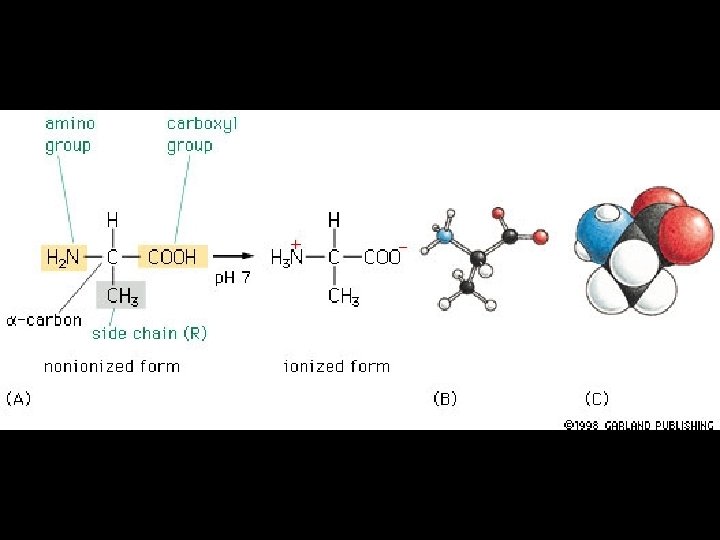
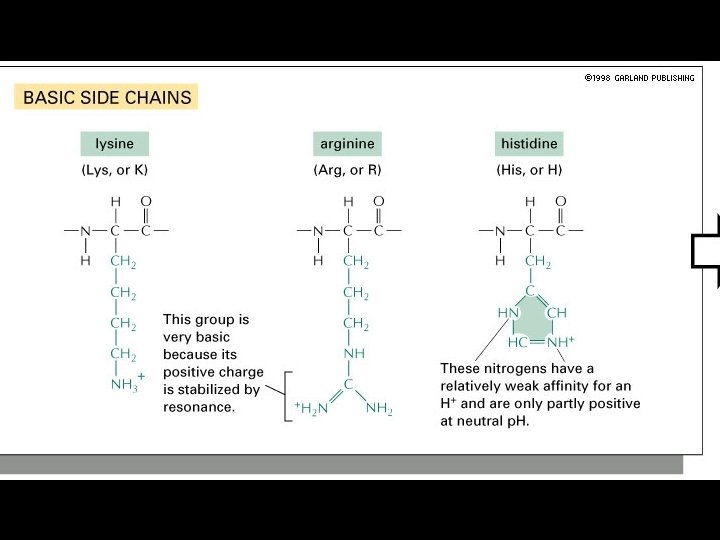
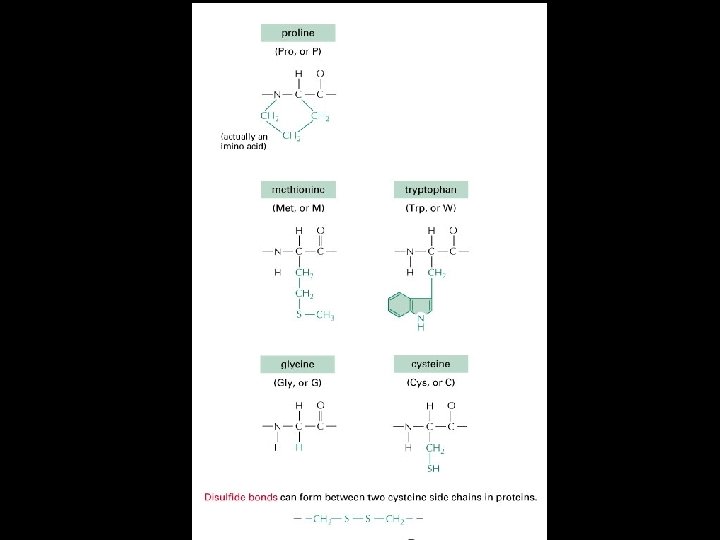
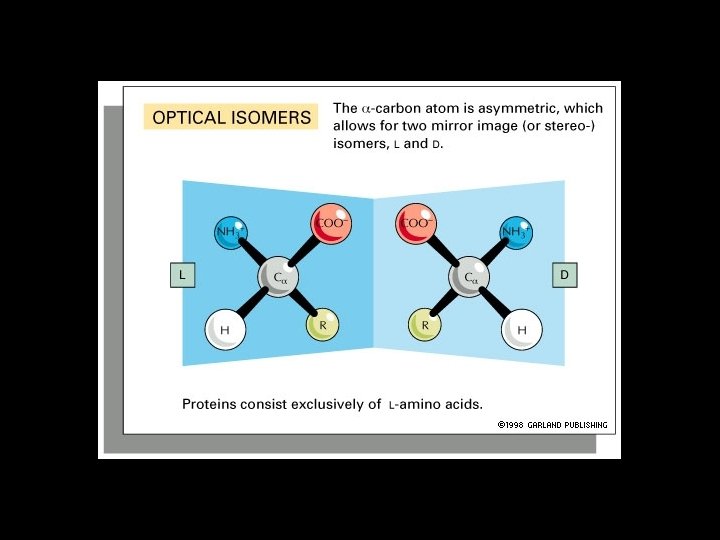
Amino Acids
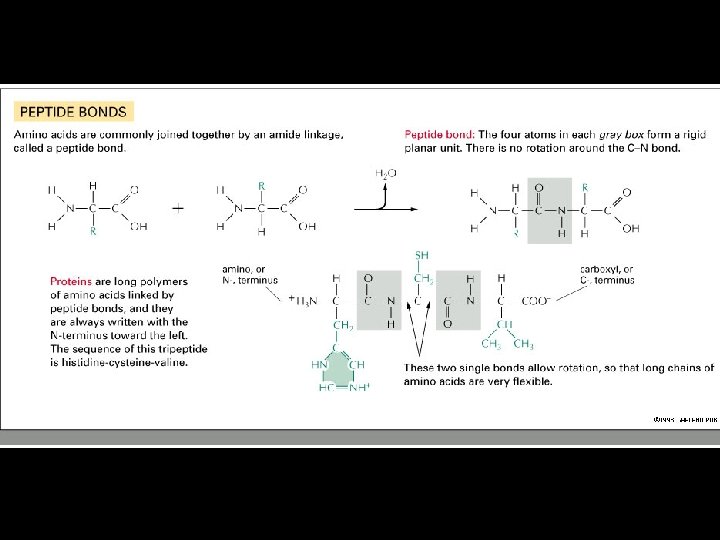
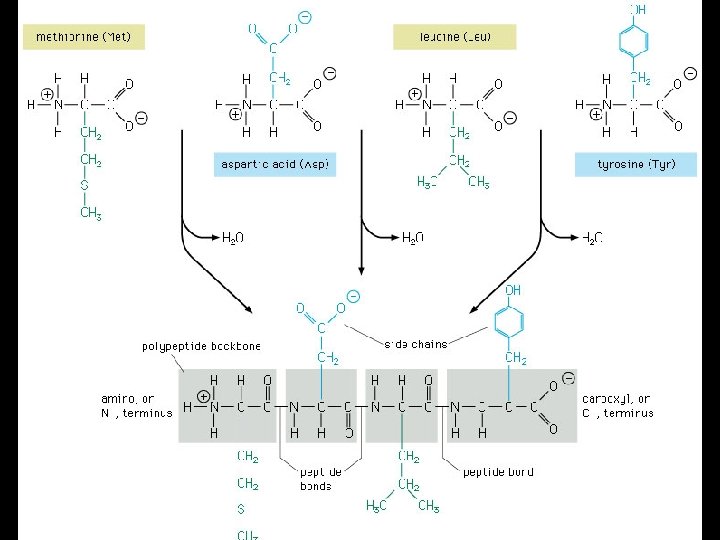
Building Molecules: The Condensation Reaction
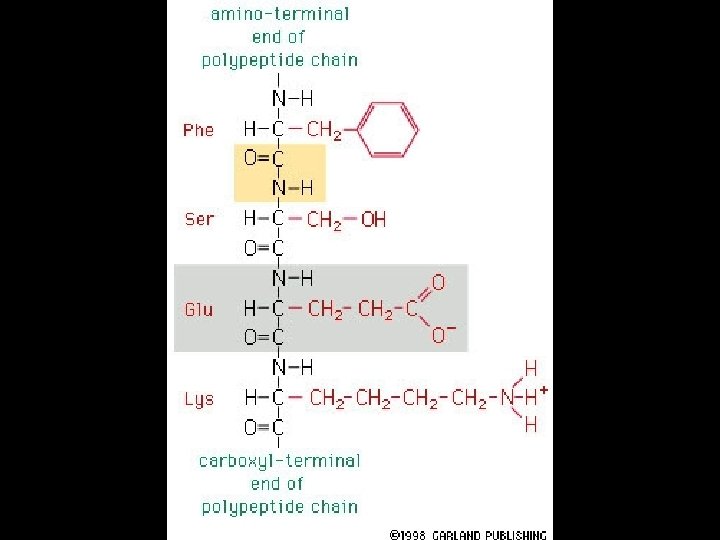
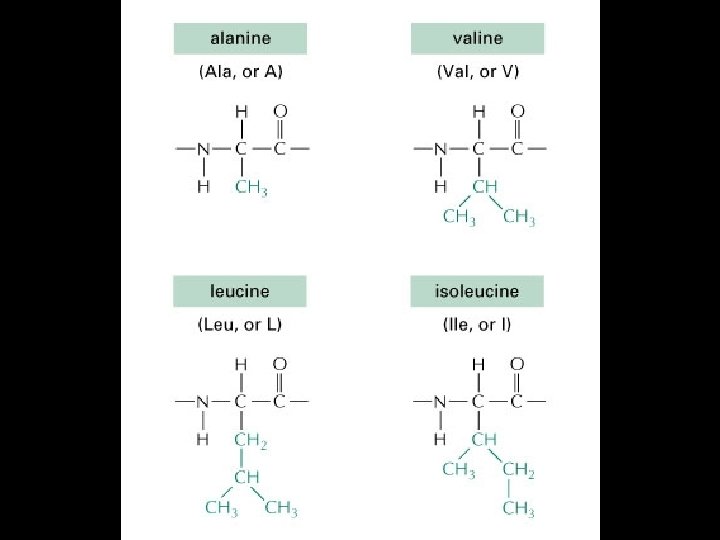
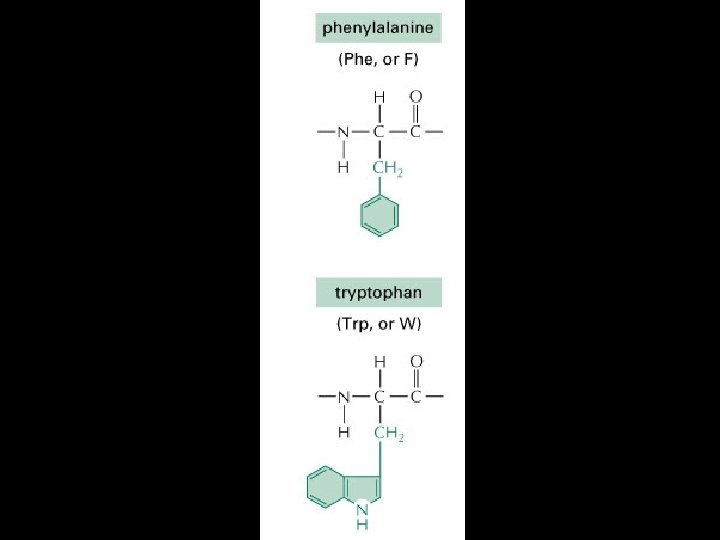
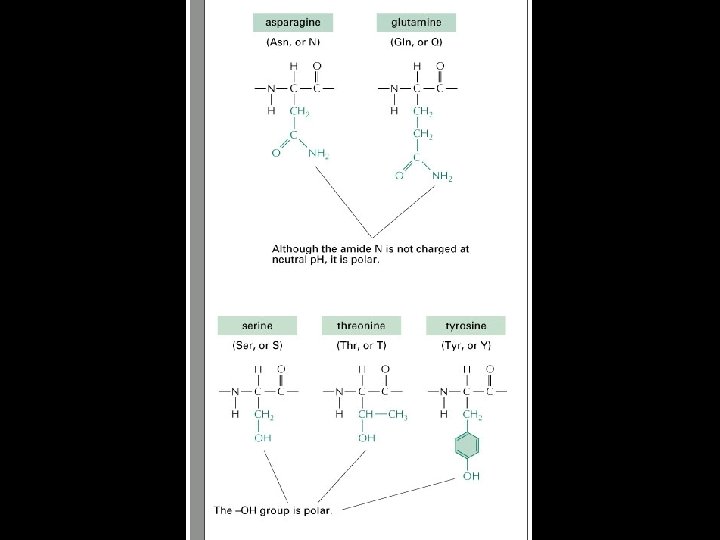
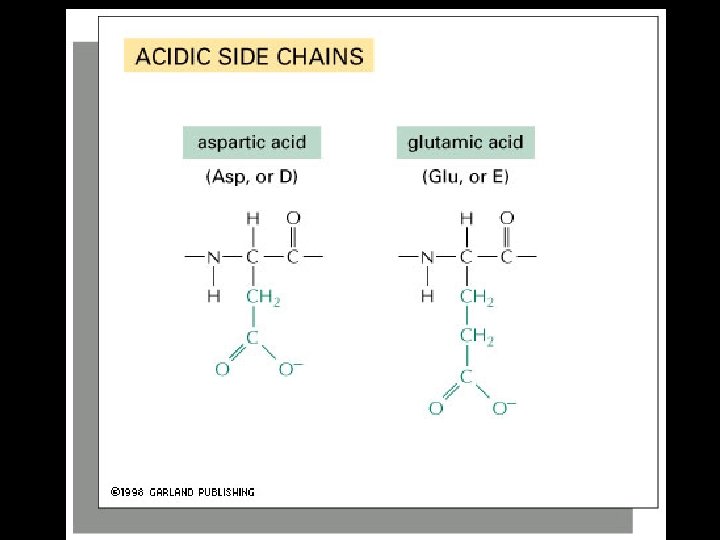
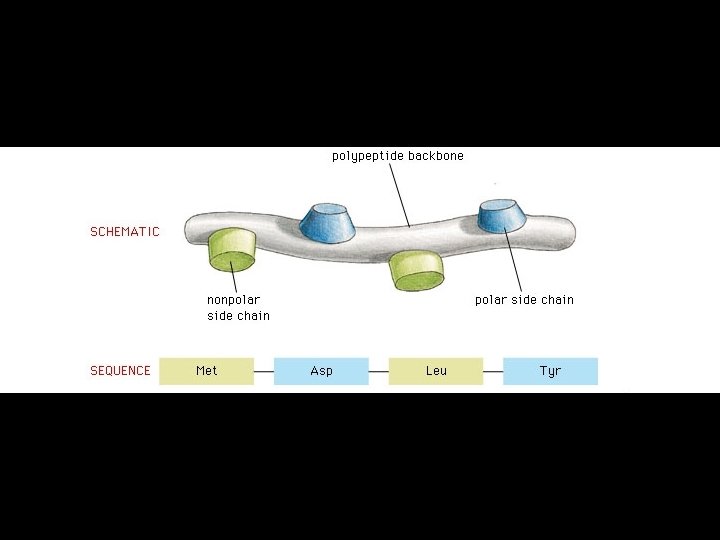
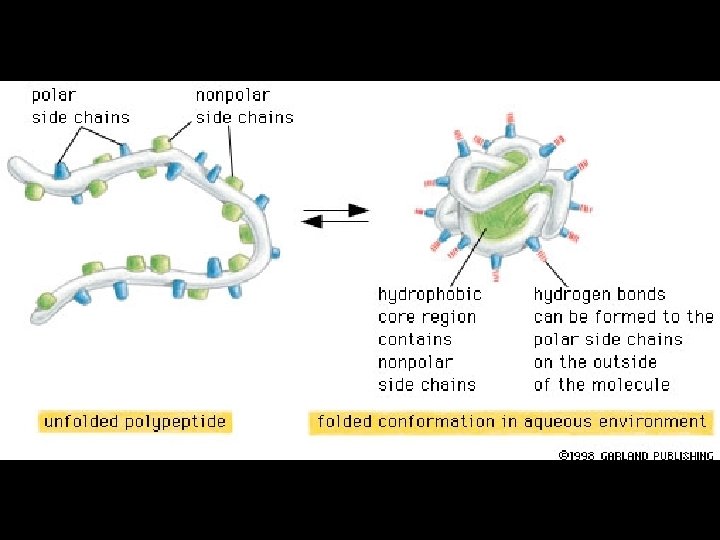
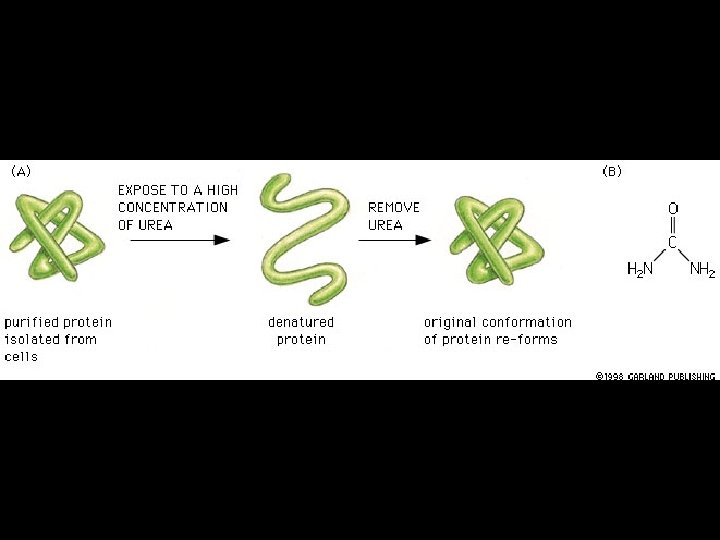
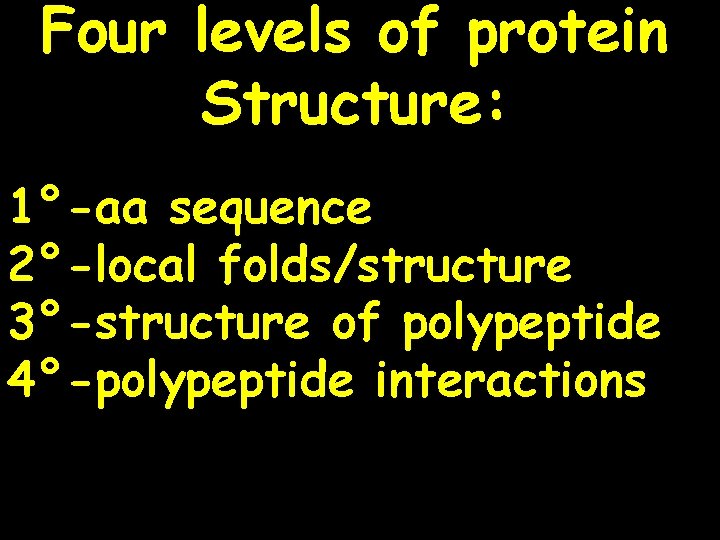
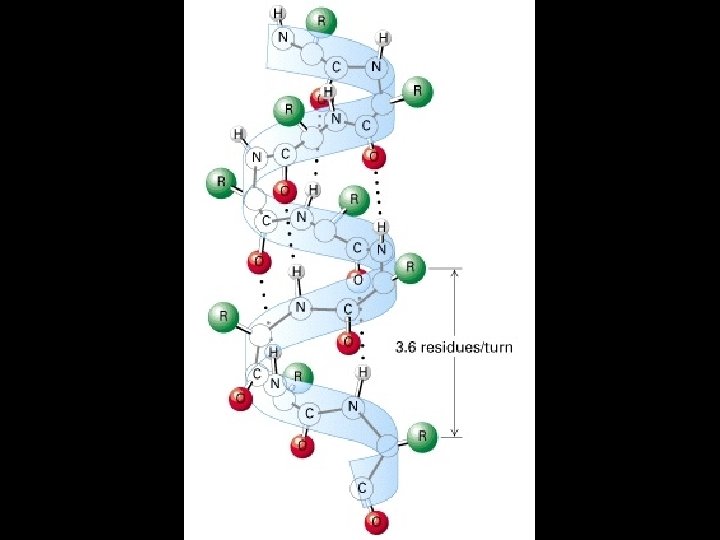
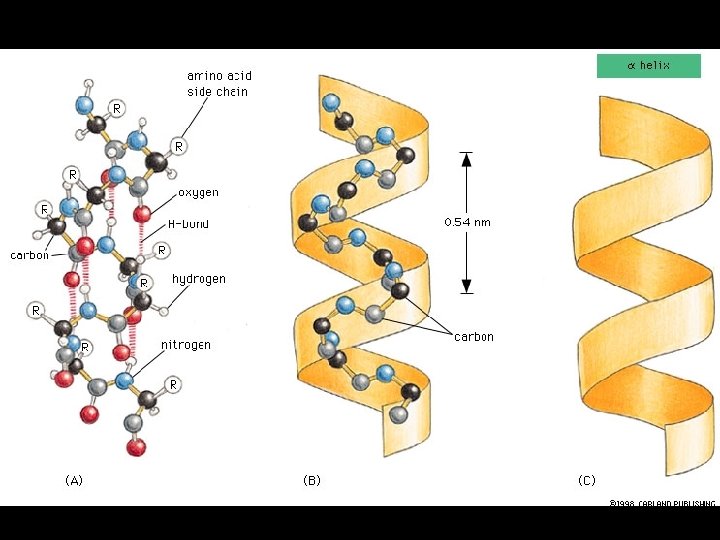
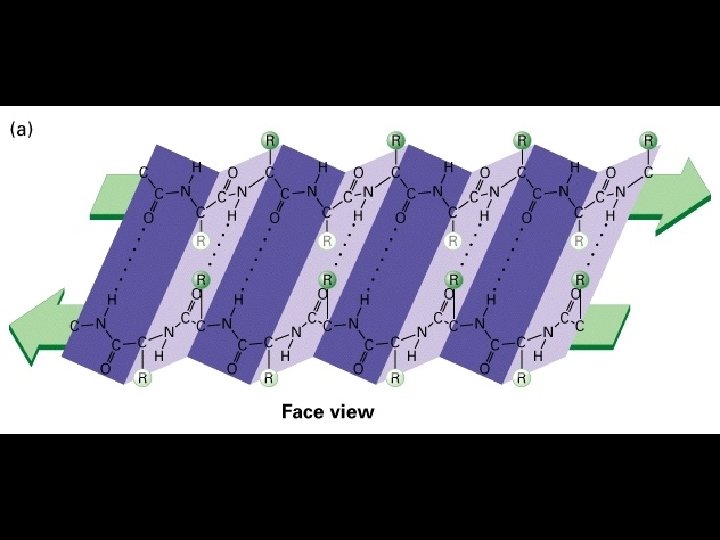
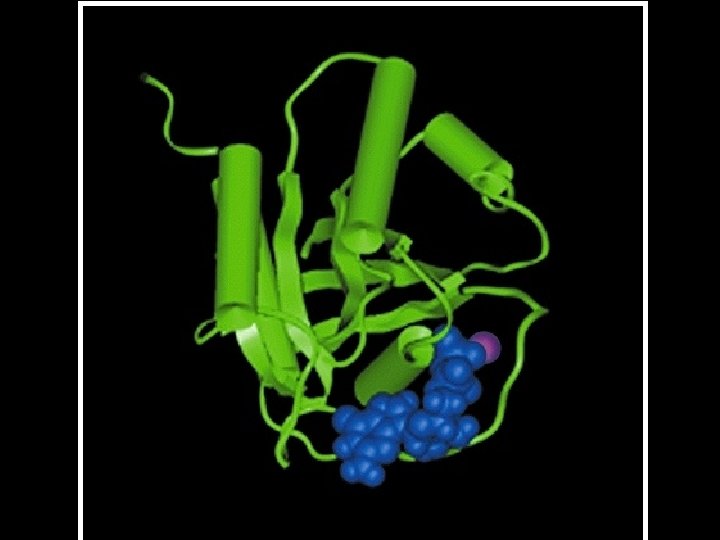
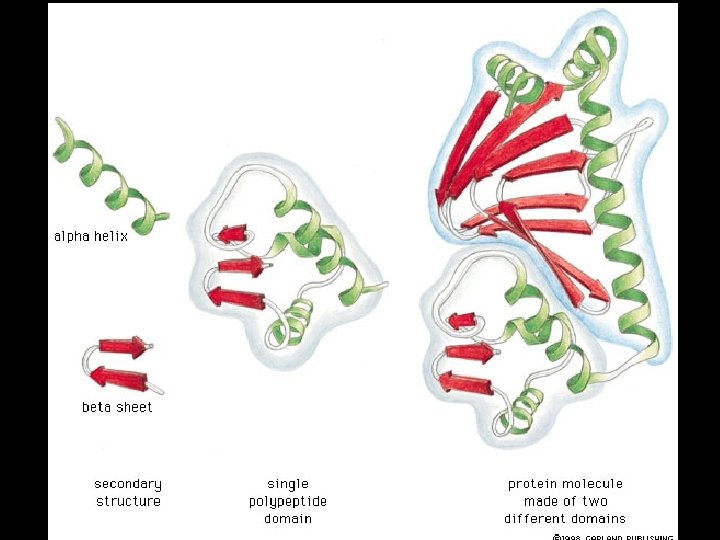
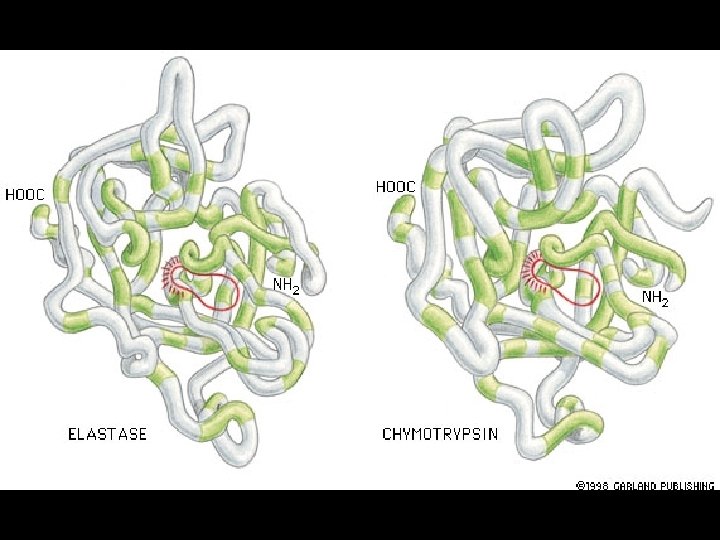
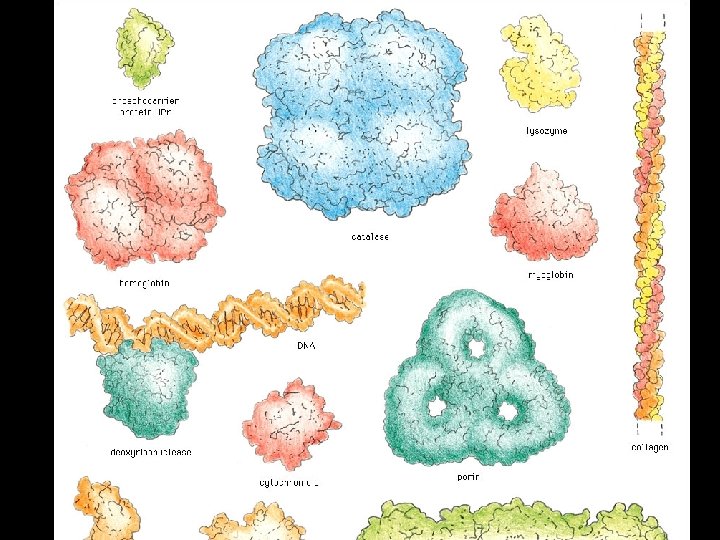
Four levels of protein Structure: 1°-aa sequence 2°-local folds/structure 3°-structure of polypeptide 4°-polypeptide interactions

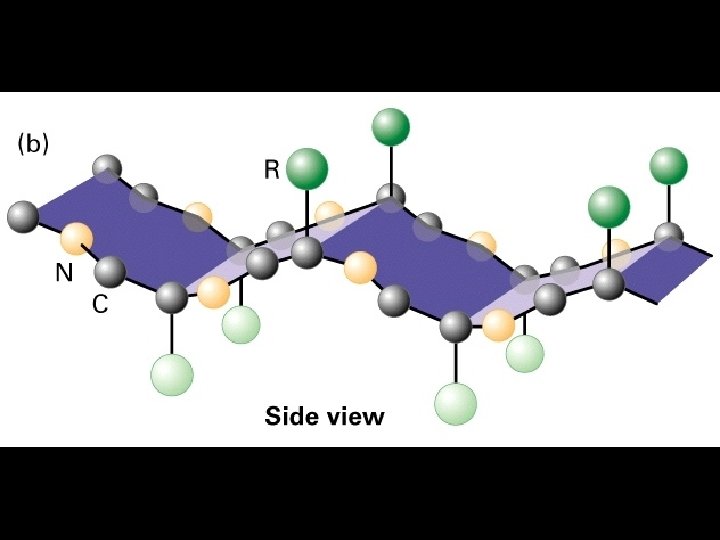
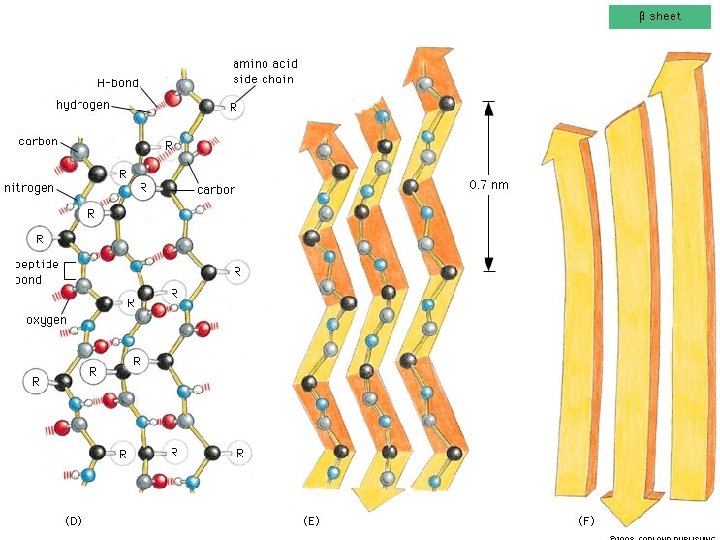
Two common folds: a-helix b-sheet
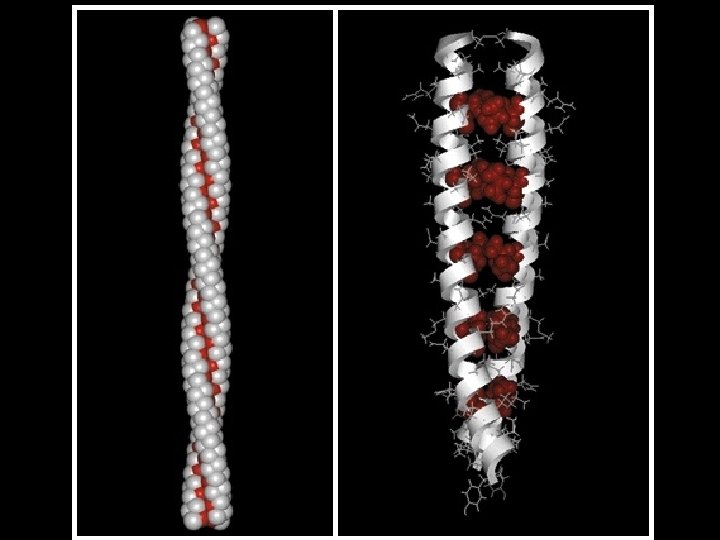
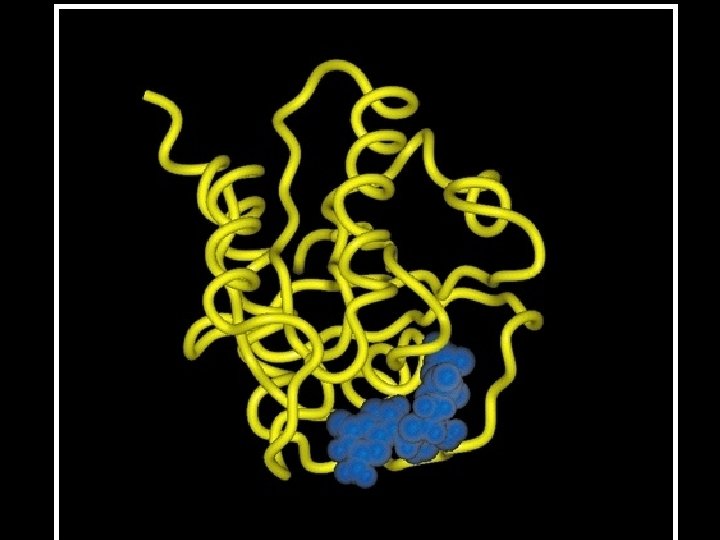
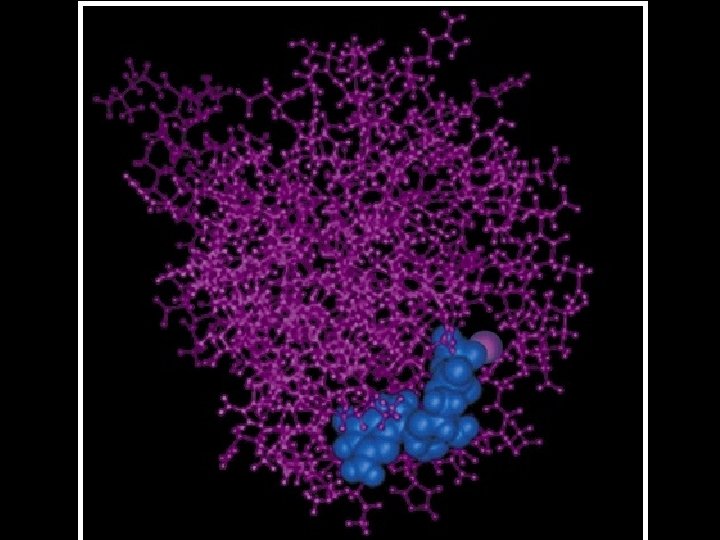
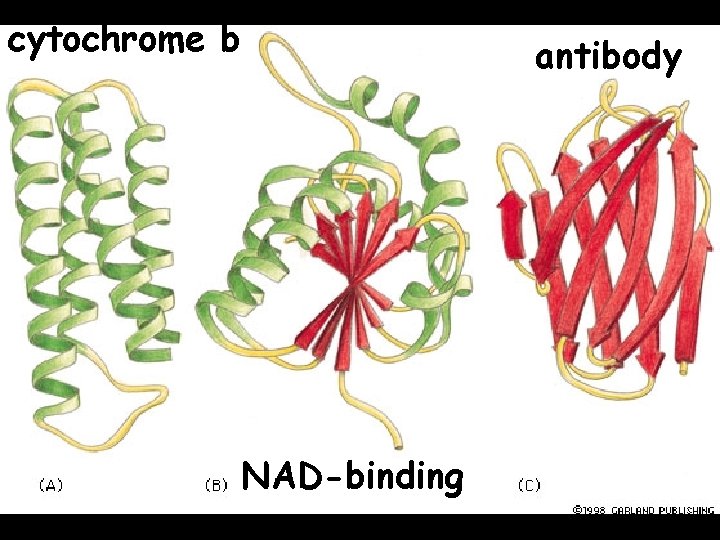
cytochrome b NAD-binding antibody

Next Class. Protein Function: How we study proteins
- Slides: 51