Molecular Structure Molecular Geometry I II III A


- Slides: 24

Molecular Structure Molecular Geometry I II III

A. VSEPR Theory Ø Valence Shell Electron Pair Repulsion Theory Ø Electron pairs orient themselves so that valence electrons are as far apart as possible

A. VSEPR Theory Ø Types of e- Pairs · Bonding pairs - form bonds · Lone pairs - nonbonding e- Lone pairs repel more strongly than bonding pairs!!!

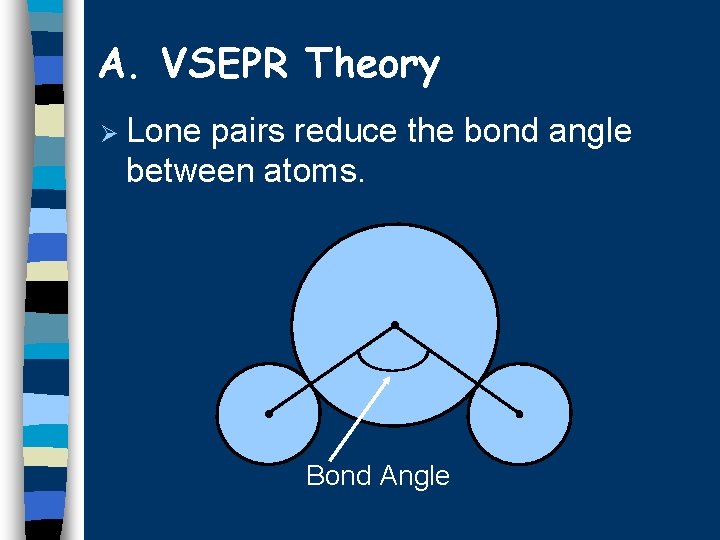
A. VSEPR Theory Ø Lone pairs reduce the bond angle between atoms. Bond Angle

B. Determining Molecular Shape Ø Draw the Lewis Diagram. Ø Count up e- pairs on central atom. · double/triple bonds = ONE pair (for shape purposes only!!) Ø Shape is determined by the # of bonding pairs and lone pairs. Know the 8 common shapes & their bond angles!

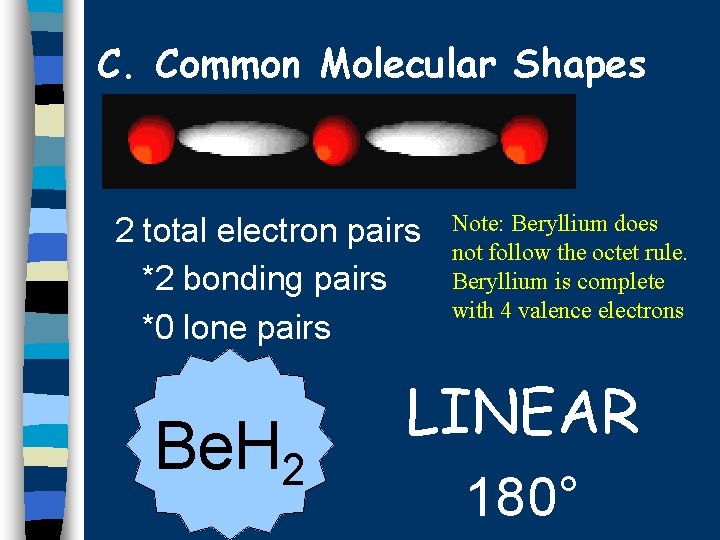
C. Common Molecular Shapes 2 total electron pairs *2 bonding pairs *0 lone pairs Be. H 2 Note: Beryllium does not follow the octet rule. Beryllium is complete with 4 valence electrons LINEAR 180°

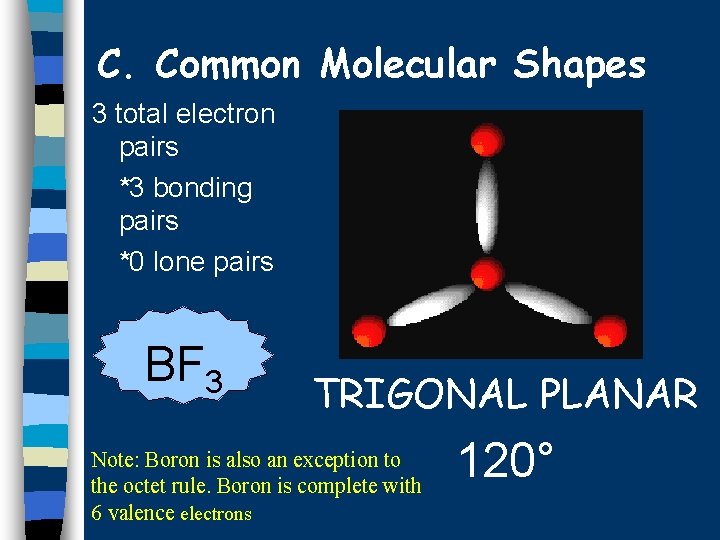
C. Common Molecular Shapes 3 total electron pairs *3 bonding pairs *0 lone pairs BF 3 TRIGONAL PLANAR Note: Boron is also an exception to the octet rule. Boron is complete with 6 valence electrons 120°

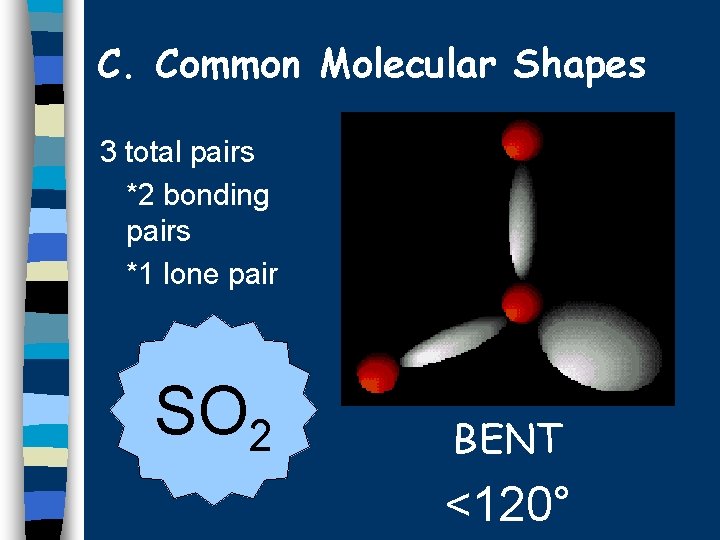
C. Common Molecular Shapes 3 total pairs *2 bonding pairs *1 lone pair SO 2 BENT <120°

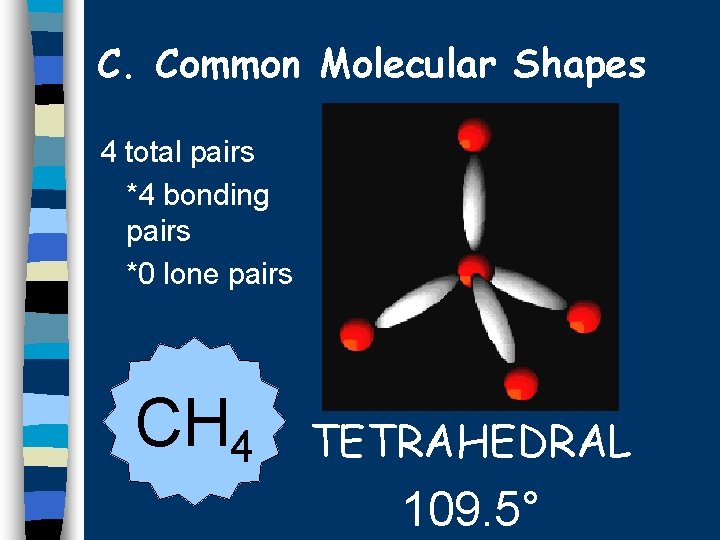
C. Common Molecular Shapes 4 total pairs *4 bonding pairs *0 lone pairs CH 4 TETRAHEDRAL 109. 5°

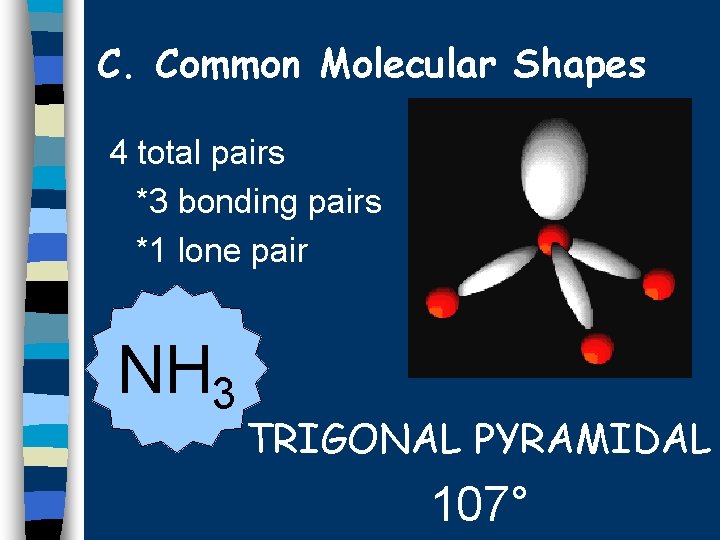
C. Common Molecular Shapes 4 total pairs *3 bonding pairs *1 lone pair NH 3 TRIGONAL PYRAMIDAL 107°

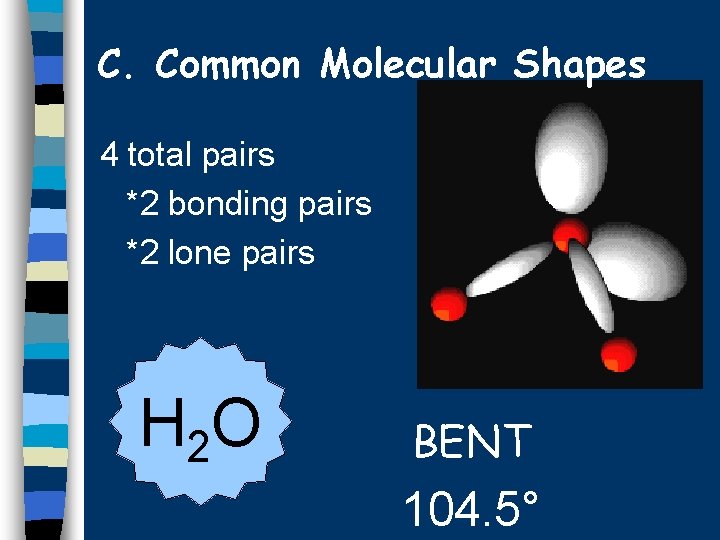
C. Common Molecular Shapes 4 total pairs *2 bonding pairs *2 lone pairs H 2 O BENT 104. 5°

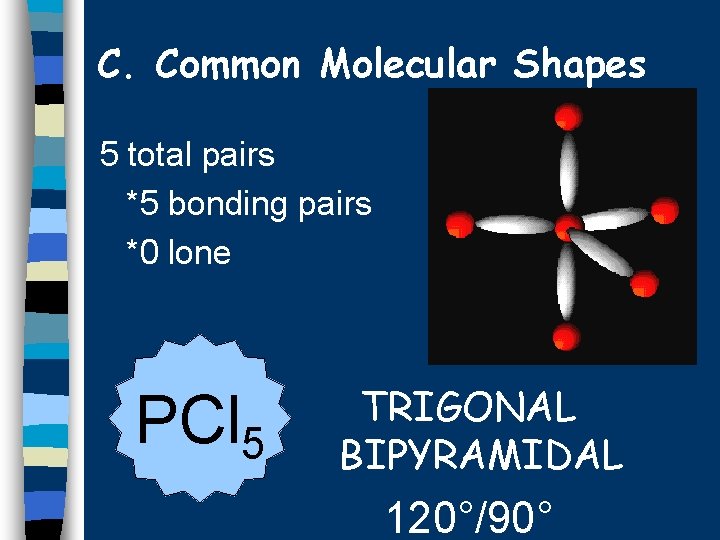
C. Common Molecular Shapes 5 total pairs *5 bonding pairs *0 lone PCl 5 TRIGONAL BIPYRAMIDAL 120°/90°

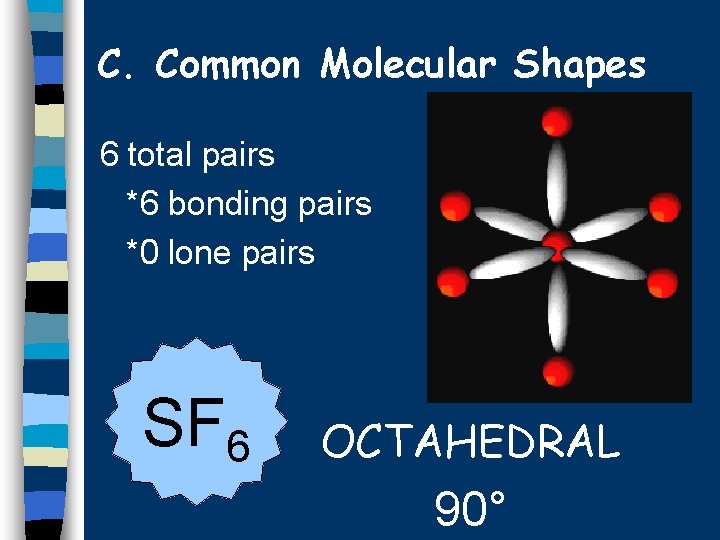
C. Common Molecular Shapes 6 total pairs *6 bonding pairs *0 lone pairs SF 6 OCTAHEDRAL 90°

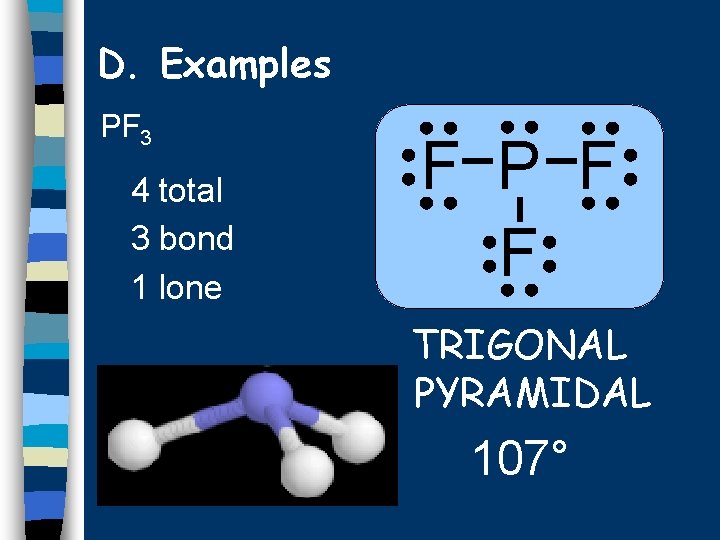
D. Examples PF 3 4 total 3 bond 1 lone F P F F TRIGONAL PYRAMIDAL 107°

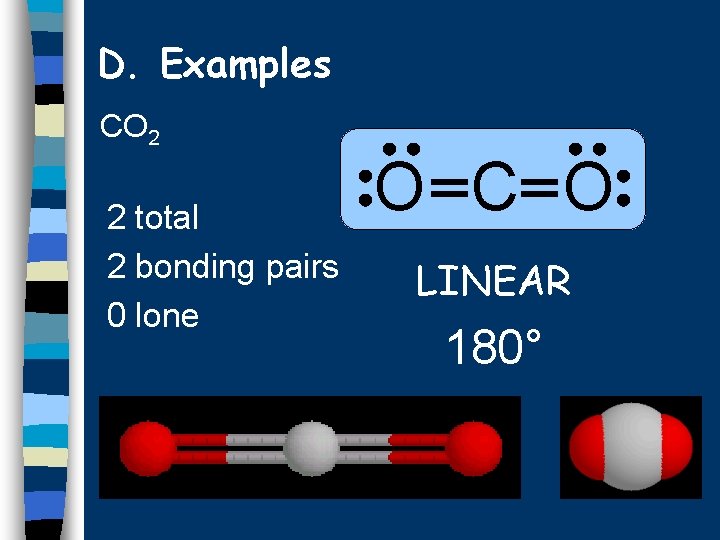
D. Examples CO 2 2 total 2 bonding pairs 0 lone O C O LINEAR 180°

Molecular Structure Molecular Polarity I II III

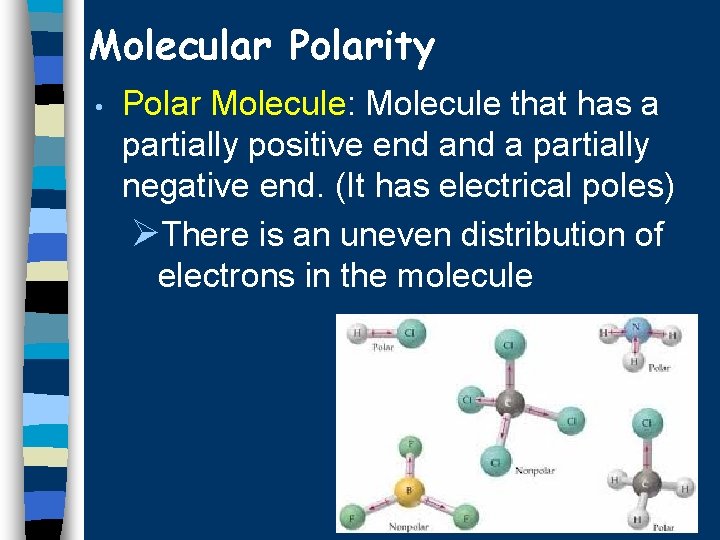
Molecular Polarity • Polar Molecule: Molecule that has a partially positive end a partially negative end. (It has electrical poles) ØThere is an uneven distribution of electrons in the molecule

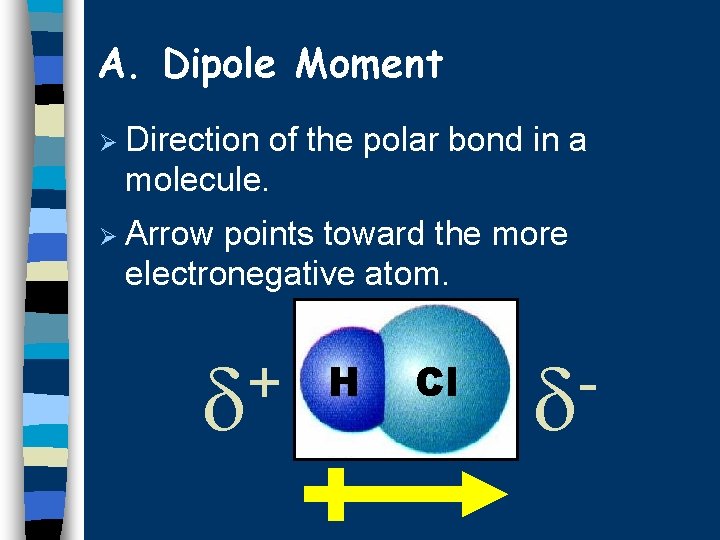
A. Dipole Moment Ø Direction of the polar bond in a molecule. Ø Arrow points toward the more electronegative atom. + H Cl

B. Determining Molecular Polarity Ø Depends on: · dipole moments · molecular shape

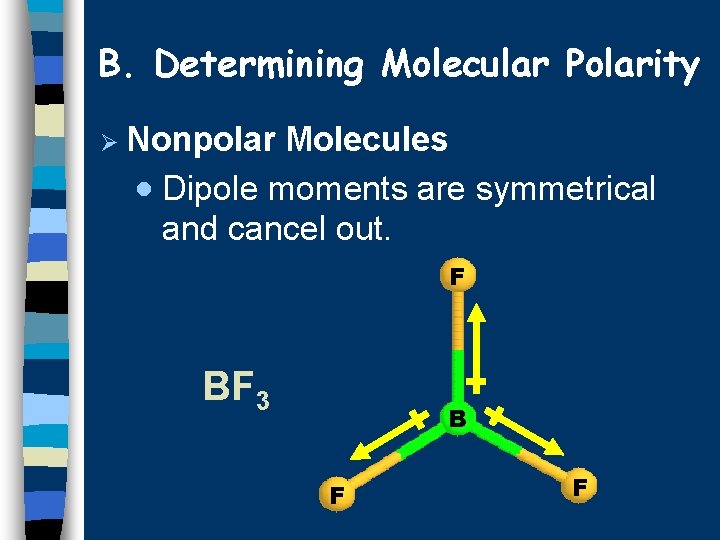
B. Determining Molecular Polarity Ø Nonpolar Molecules · Dipole moments are symmetrical and cancel out. F BF 3 B F F

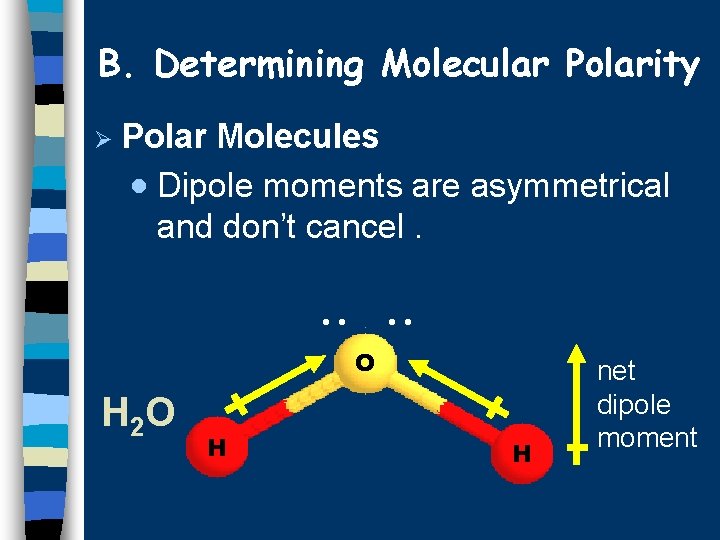
B. Determining Molecular Polarity Ø Polar Molecules · Dipole moments are asymmetrical and don’t cancel. . . O H 2 O H H net dipole moment

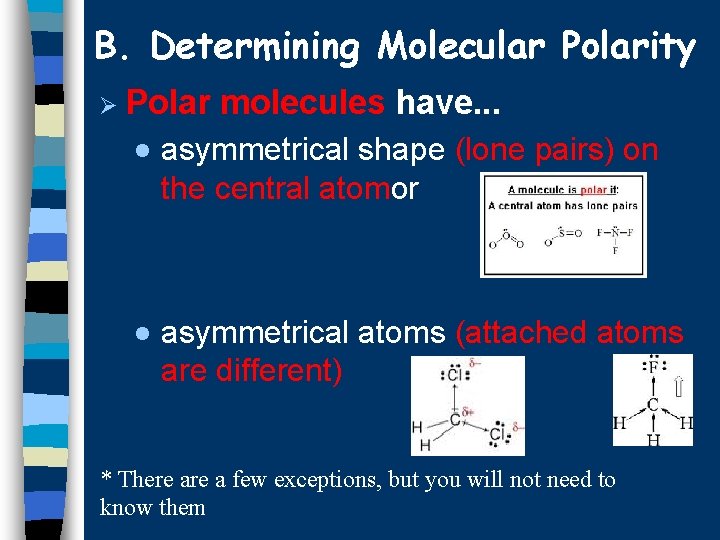
B. Determining Molecular Polarity Ø Polar molecules have. . . · asymmetrical shape (lone pairs) on the central atomor · asymmetrical atoms (attached atoms are different) * There a few exceptions, but you will not need to know them

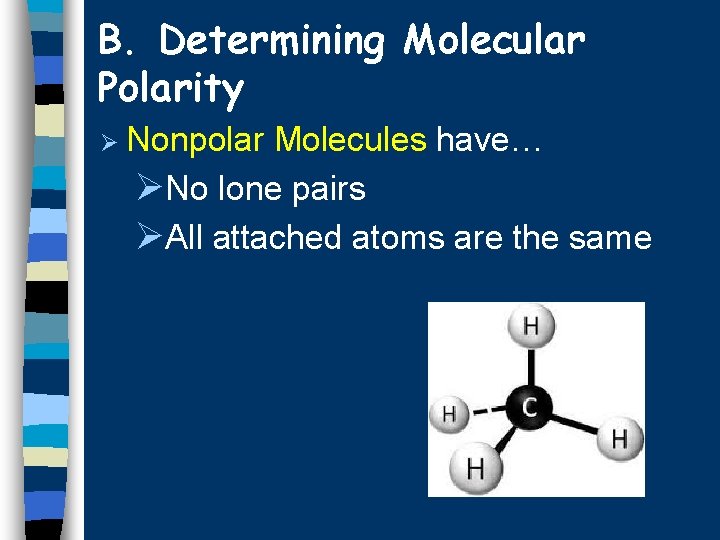
B. Determining Molecular Polarity Ø Nonpolar Molecules have… ØNo lone pairs ØAll attached atoms are the same

§ http: //highered. mheducation. com/site s/dl/free/0073402656/566295/Polarity _of_Molecules. swf