Molecular Spectroscopy 1 1 Electromagnetic Spectrum Electromagnetic radiation




- Slides: 19

Molecular Spectroscopy 1

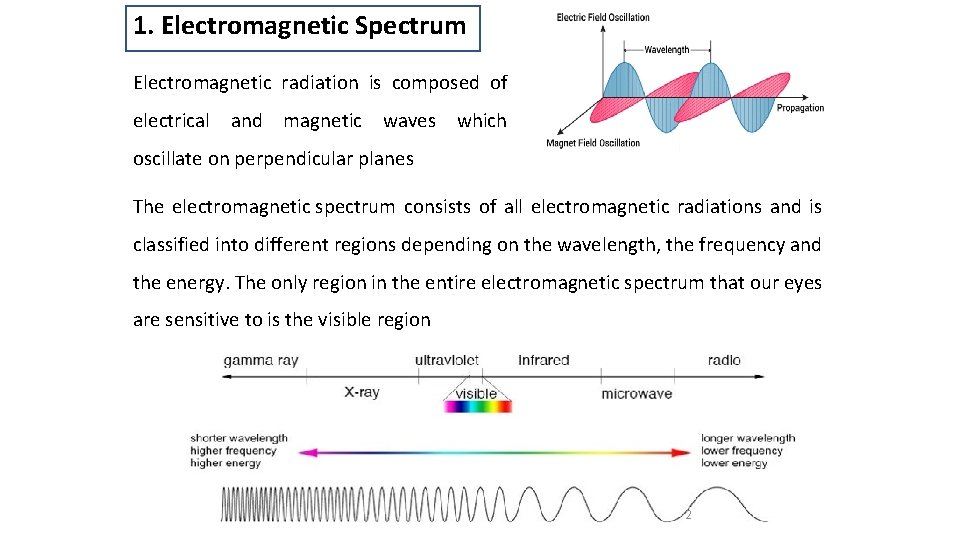
1. Electromagnetic Spectrum Electromagnetic radiation is composed of electrical and magnetic waves which oscillate on perpendicular planes The electromagnetic spectrum consists of all electromagnetic radiations and is classified into different regions depending on the wavelength, the frequency and the energy. The only region in the entire electromagnetic spectrum that our eyes are sensitive to is the visible region 2

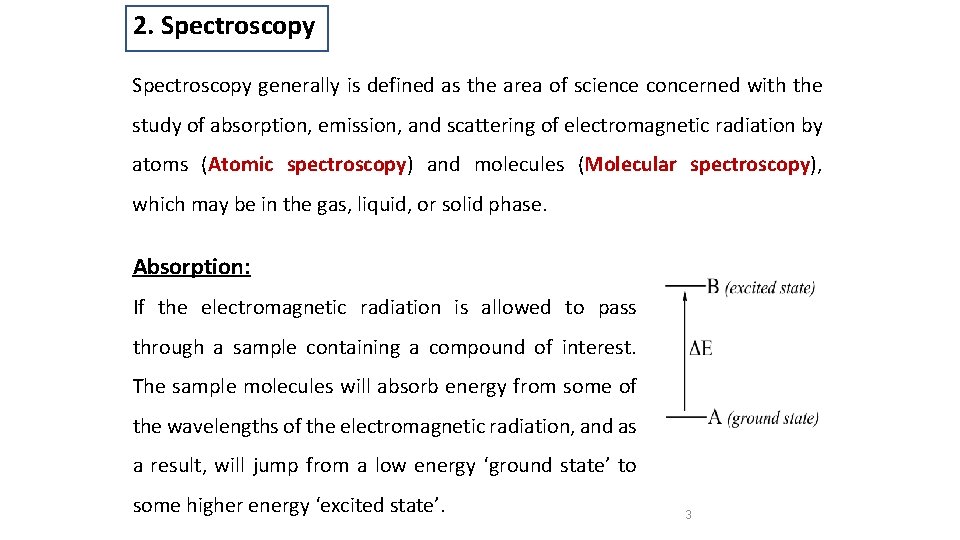
2. Spectroscopy generally is defined as the area of science concerned with the study of absorption, emission, and scattering of electromagnetic radiation by atoms (Atomic spectroscopy) and molecules (Molecular spectroscopy), which may be in the gas, liquid, or solid phase. Absorption: If the electromagnetic radiation is allowed to pass through a sample containing a compound of interest. The sample molecules will absorb energy from some of the wavelengths of the electromagnetic radiation, and as a result, will jump from a low energy ‘ground state’ to some higher energy ‘excited state’. 3

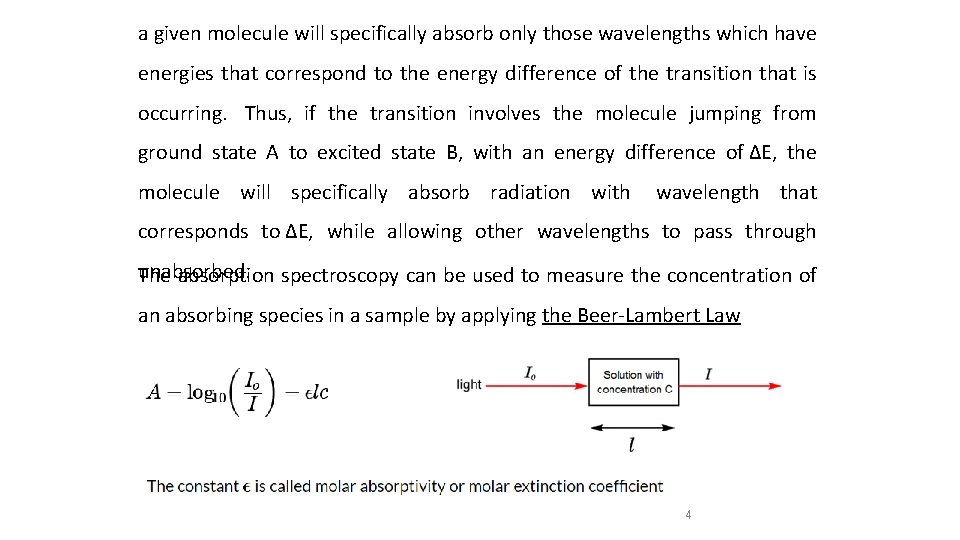
a given molecule will specifically absorb only those wavelengths which have energies that correspond to the energy difference of the transition that is occurring. Thus, if the transition involves the molecule jumping from ground state A to excited state B, with an energy difference of ΔE, the molecule will specifically absorb radiation with wavelength that corresponds to ΔE, while allowing other wavelengths to pass through unabsorbed. The absorption spectroscopy can be used to measure the concentration of an absorbing species in a sample by applying the Beer-Lambert Law 4

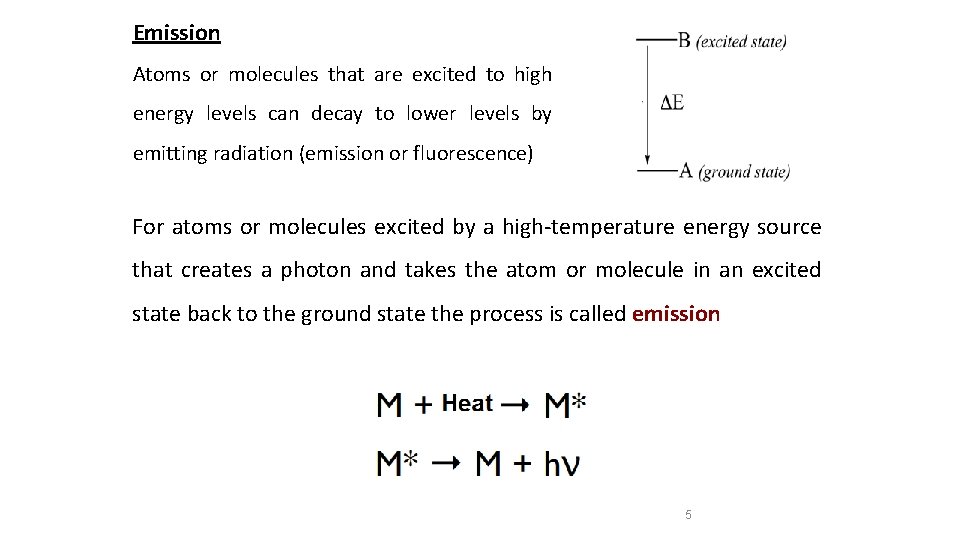
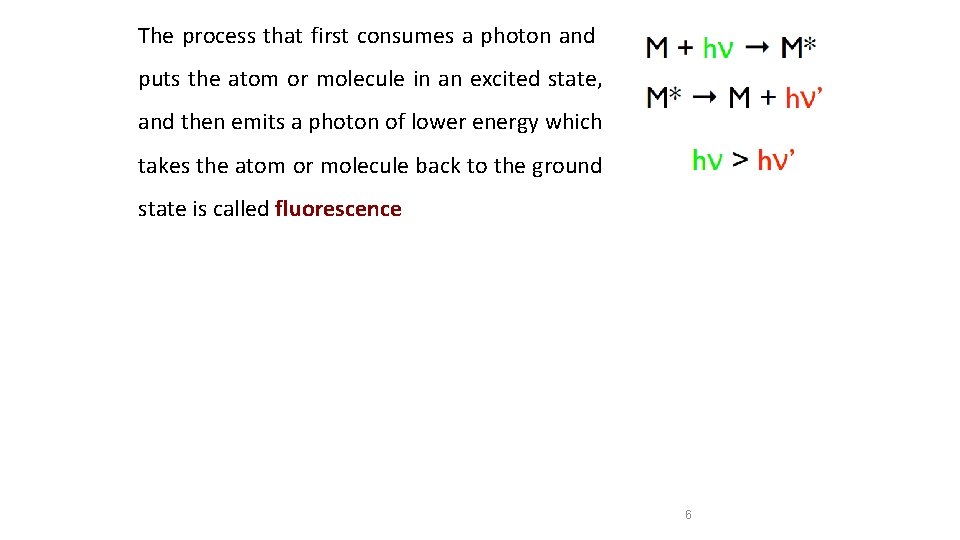
Emission Atoms or molecules that are excited to high energy levels can decay to lower levels by emitting radiation (emission or fluorescence) For atoms or molecules excited by a high-temperature energy source that creates a photon and takes the atom or molecule in an excited state back to the ground state the process is called emission 5

The process that first consumes a photon and puts the atom or molecule in an excited state, and then emits a photon of lower energy which takes the atom or molecule back to the ground state is called fluorescence 6

Scattering When electromagnetic radiation passes through matter, most of the radiation continues in its original direction but a small fraction is scattered in other directions. Two general categories of scattering are recognized. In elastic scattering, radiation is first absorbed by the particles and then emitted without undergoing change in the radiation’s energy. When the radiation emerges with a change in energy, the scattering is said to be inelastic. 7

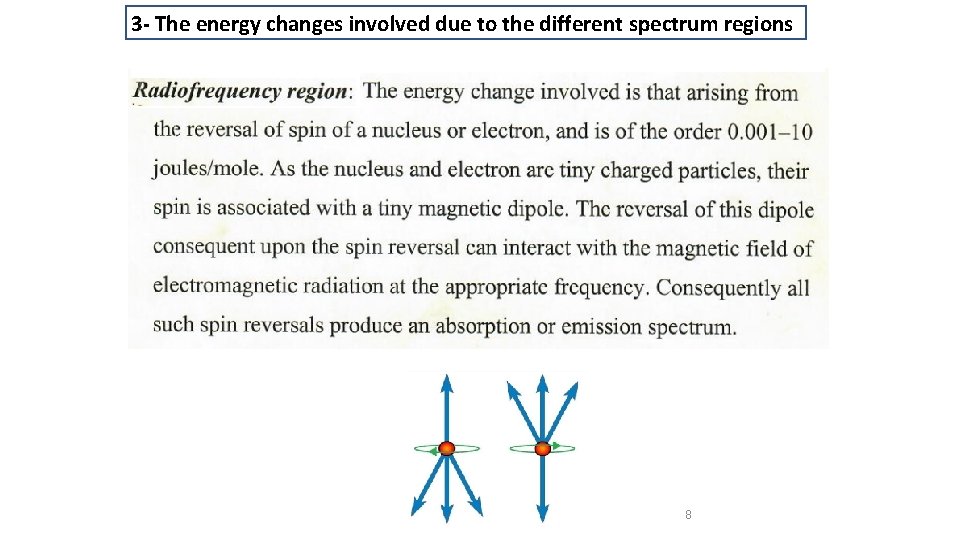
3 - The energy changes involved due to the different spectrum regions 8

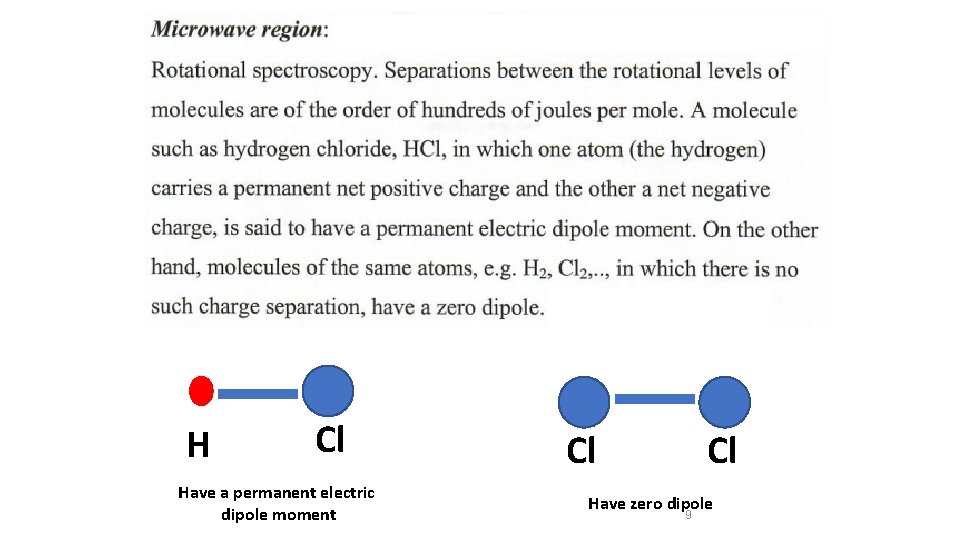
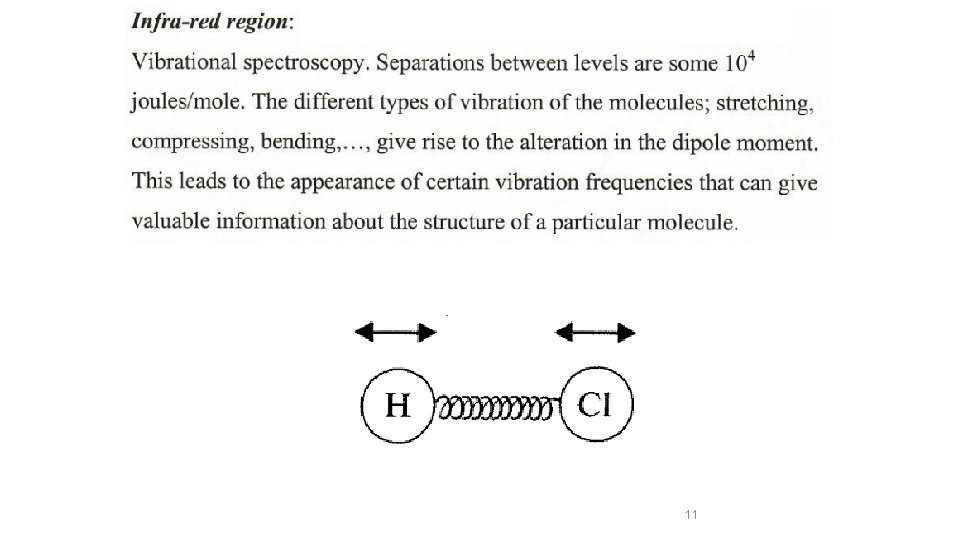
H Cl Have a permanent electric dipole moment Cl Cl Have zero dipole 9

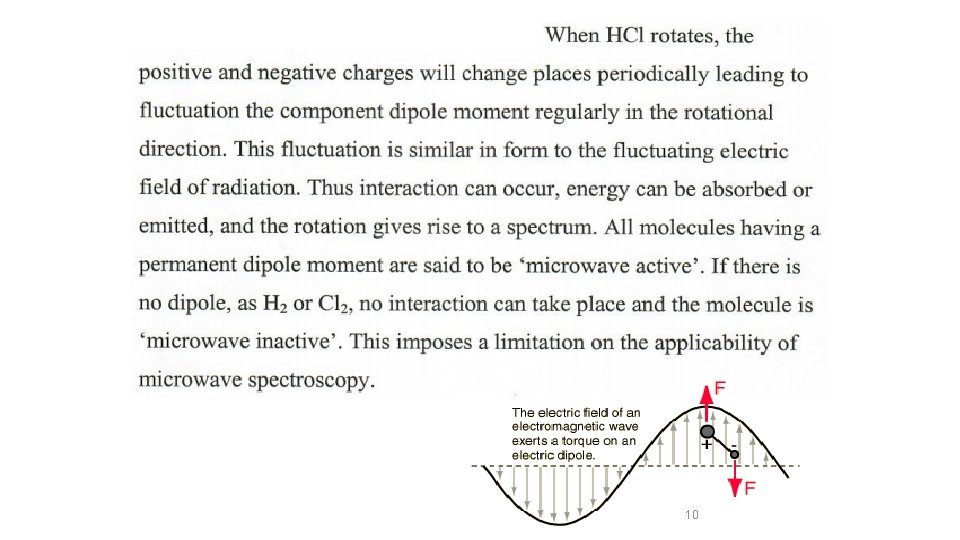
10

11

12

4 - Categories of thermal energies of molecules of gases 13

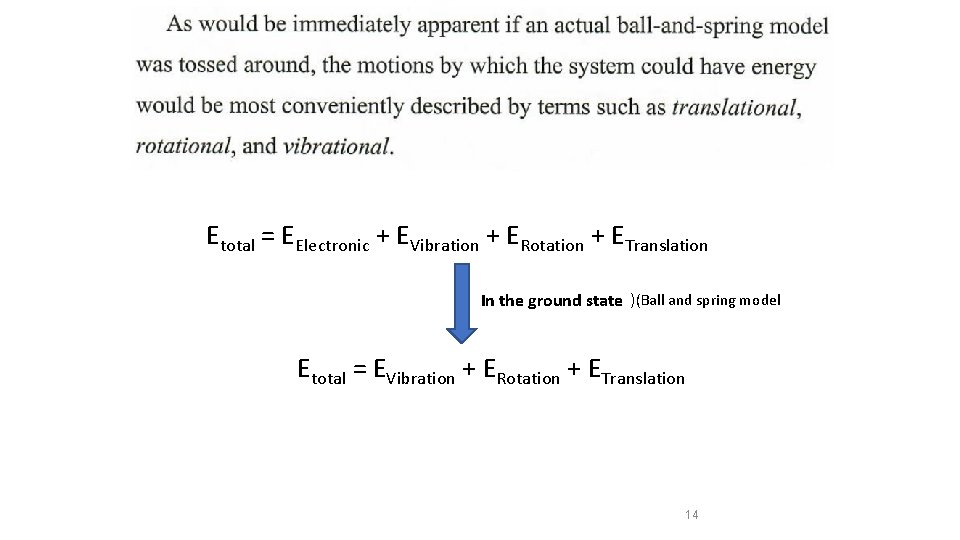
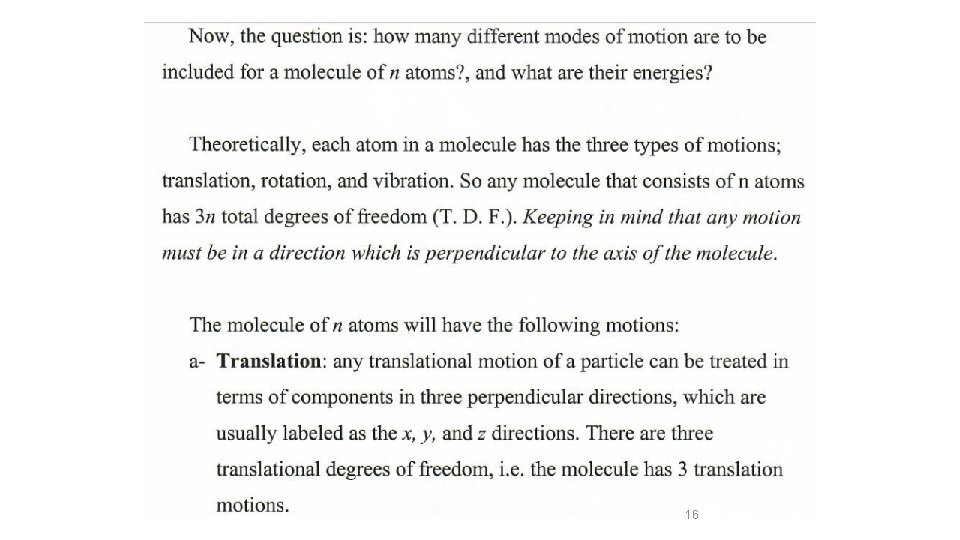
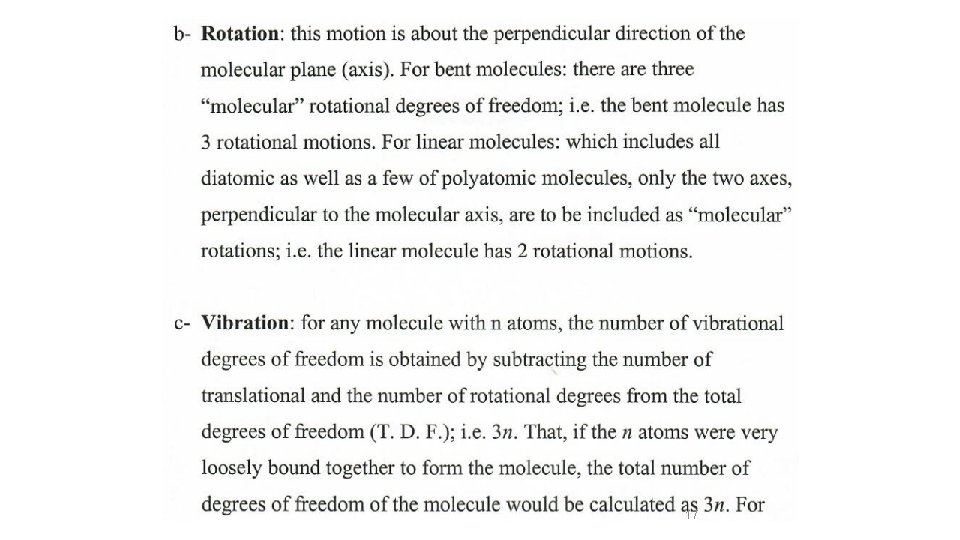
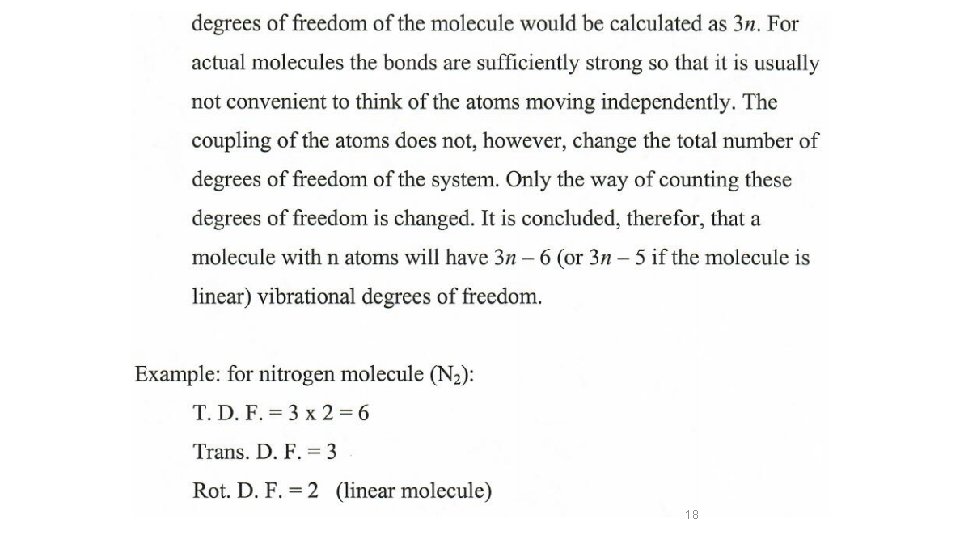
Etotal = EElectronic + EVibration + ERotation + ETranslation In the ground state )(Ball and spring model Etotal = EVibration + ERotation + ETranslation 14

15

16

17

18

19