Molecular Shapes and Polarity 2 Valence Shell Electron

- Slides: 8

Molecular Shapes and Polarity 2

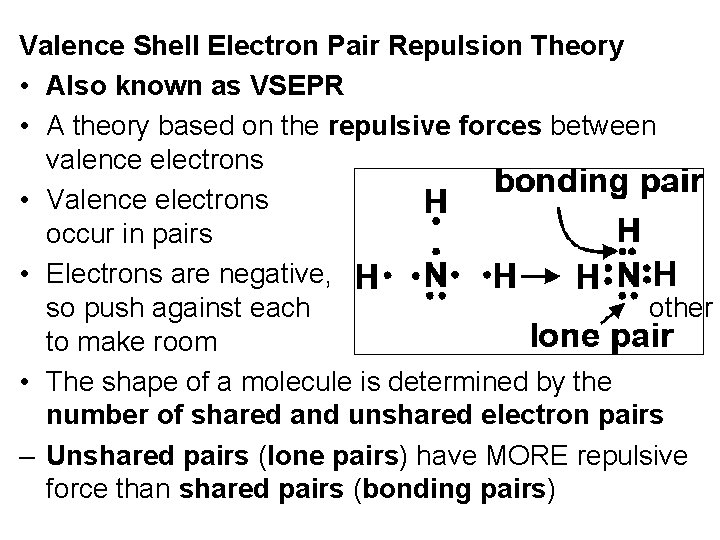
Valence Shell Electron Pair Repulsion Theory • Also known as VSEPR • A theory based on the repulsive forces between valence electrons • Valence electrons occur in pairs • Electrons are negative, so push against each other to make room • The shape of a molecule is determined by the number of shared and unshared electron pairs – Unshared pairs (lone pairs) have MORE repulsive force than shared pairs (bonding pairs)

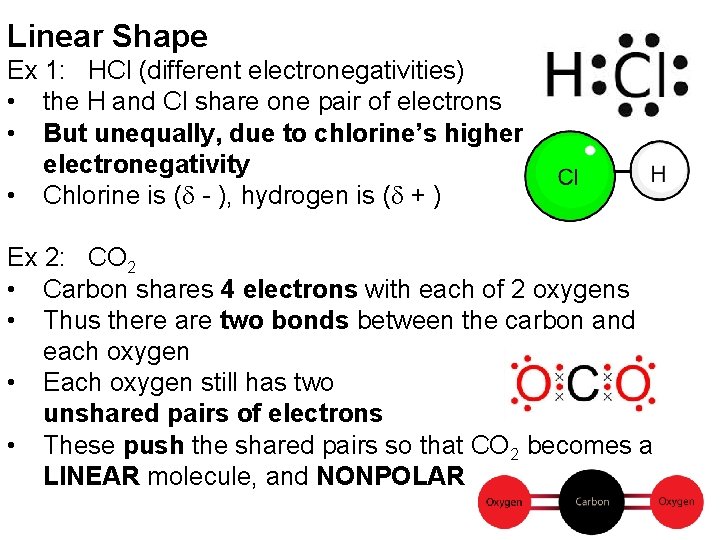
Linear Shape Ex 1: HCl (different electronegativities) • the H and Cl share one pair of electrons • But unequally, due to chlorine’s higher electronegativity • Chlorine is (d - ), hydrogen is (d + ) Ex 2: CO 2 • Carbon shares 4 electrons with each of 2 oxygens • Thus there are two bonds between the carbon and each oxygen • Each oxygen still has two unshared pairs of electrons • These push the shared pairs so that CO 2 becomes a LINEAR molecule, and NONPOLAR

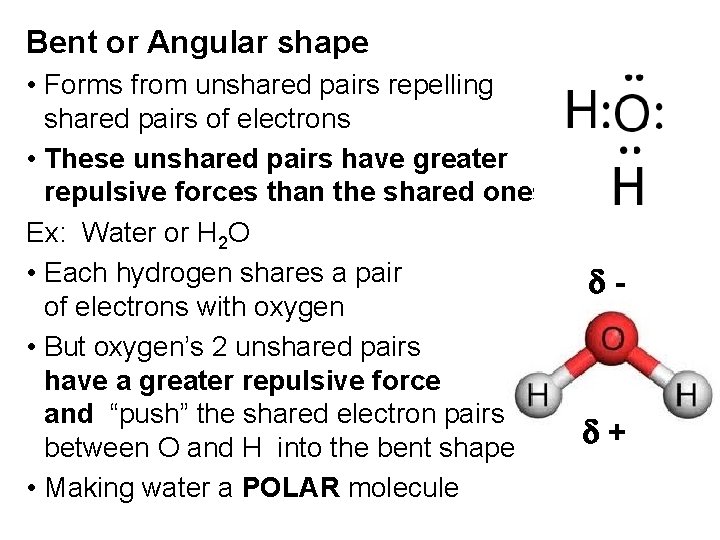
Bent or Angular shape • Forms from unshared pairs repelling shared pairs of electrons • These unshared pairs have greater repulsive forces than the shared ones Ex: Water or H 2 O • Each hydrogen shares a pair of electrons with oxygen • But oxygen’s 2 unshared pairs have a greater repulsive force and “push” the shared electron pairs between O and H into the bent shape • Making water a POLAR molecule d- d+

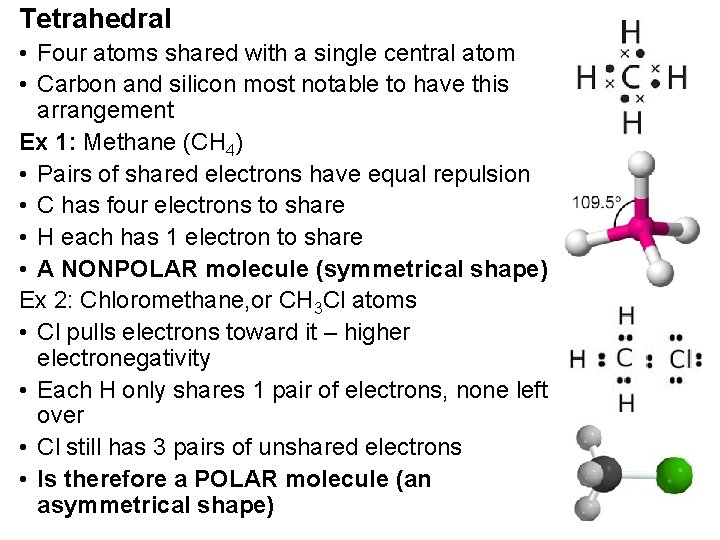
Tetrahedral • Four atoms shared with a single central atom • Carbon and silicon most notable to have this arrangement Ex 1: Methane (CH 4) • Pairs of shared electrons have equal repulsion • C has four electrons to share • H each has 1 electron to share • A NONPOLAR molecule (symmetrical shape) Ex 2: Chloromethane, or CH 3 Cl atoms • Cl pulls electrons toward it – higher electronegativity • Each H only shares 1 pair of electrons, none left over • Cl still has 3 pairs of unshared electrons • Is therefore a POLAR molecule (an asymmetrical shape)

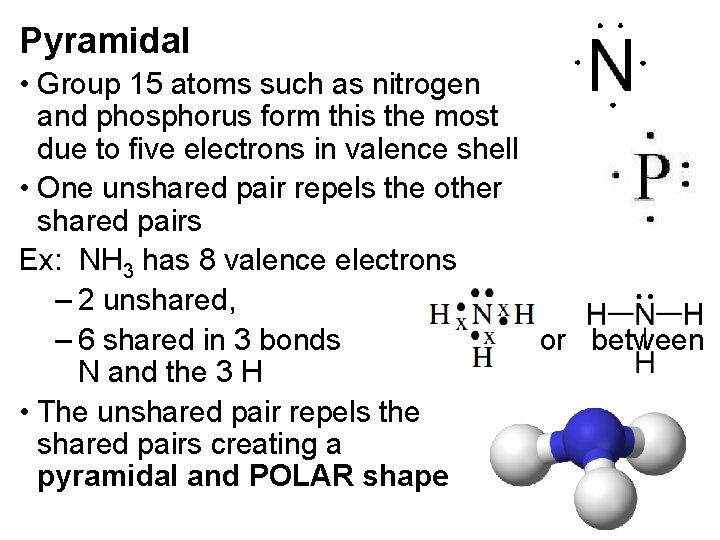
Pyramidal • Group 15 atoms such as nitrogen and phosphorus form this the most due to five electrons in valence shell • One unshared pair repels the other shared pairs Ex: NH 3 has 8 valence electrons – 2 unshared, – 6 shared in 3 bonds or between N and the 3 H • The unshared pair repels the shared pairs creating a pyramidal and POLAR shape

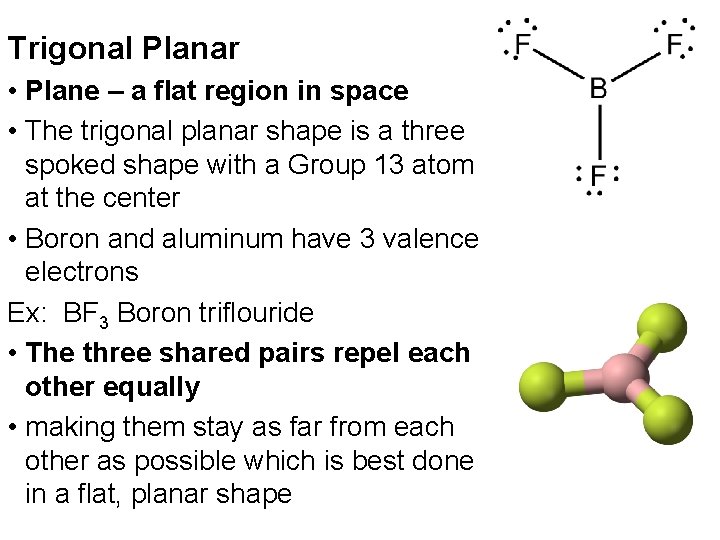
Trigonal Planar • Plane – a flat region in space • The trigonal planar shape is a three spoked shape with a Group 13 atom at the center • Boron and aluminum have 3 valence electrons Ex: BF 3 Boron triflouride • The three shared pairs repel each other equally • making them stay as far from each other as possible which is best done in a flat, planar shape

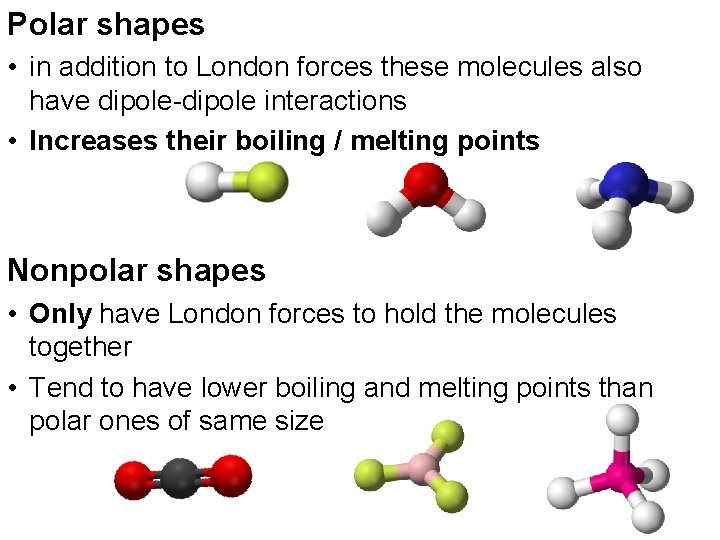
Polar shapes • in addition to London forces these molecules also have dipole-dipole interactions • Increases their boiling / melting points Nonpolar shapes • Only have London forces to hold the molecules together • Tend to have lower boiling and melting points than polar ones of same size