Molecular Shape Things to remember shape of molecule
Molecular Shape
Things to remember shape of molecule: – determined by location of nuclei – nuclei go to certain locations because of its electron pairs

Use the Lewis Structure • Lewis structure is 2 -D, but can help figure out 3 -D shape
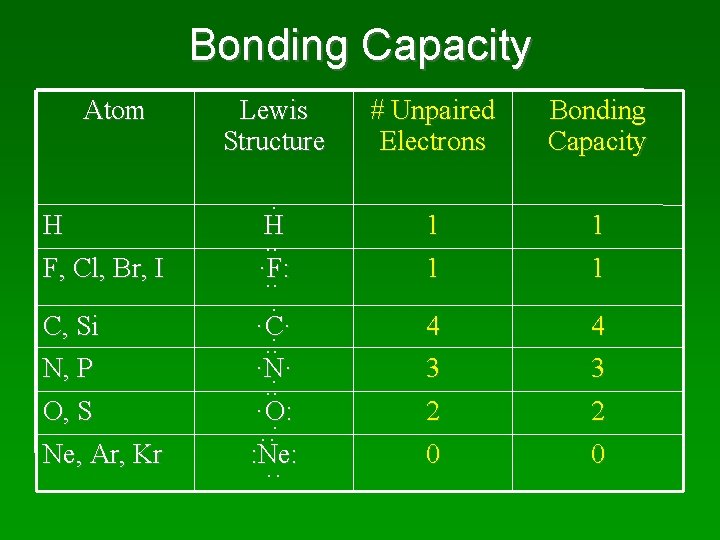
Bonding Capacity Atom H F, Cl, Br, I C, Si N, P O, S Ne, Ar, Kr Lewis Structure. H. . ·F: . . . ·C·. . . ·N·. . . ·O: ··· : Ne: ·· # Unpaired Electrons Bonding Capacity 1 1 4 3 2 2 0 0
Molecular Shape • Determined by overlap of orbitals • Shape determined by two factors: 1. total # atoms & 2. # e- pairs in different locations on central atom Classify electron pairs as bonding or nonbonding
Molecular Shape & VSEPR • Electron pairs repel each other: –want to be as far apart from each other as can be • Nonbonding pairs take up a little more room than bonding pairs
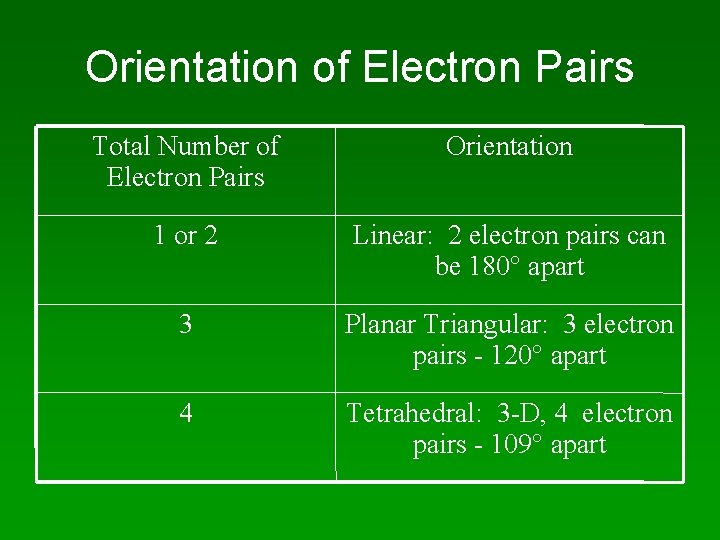
Orientation of Electron Pairs Total Number of Electron Pairs Orientation 1 or 2 Linear: 2 electron pairs can be 180 apart 3 Planar Triangular: 3 electron pairs - 120 apart 4 Tetrahedral: 3 -D, 4 electron pairs - 109 apart

2 -Atom Molecules • Atoms located right next to each other • linear molecules!
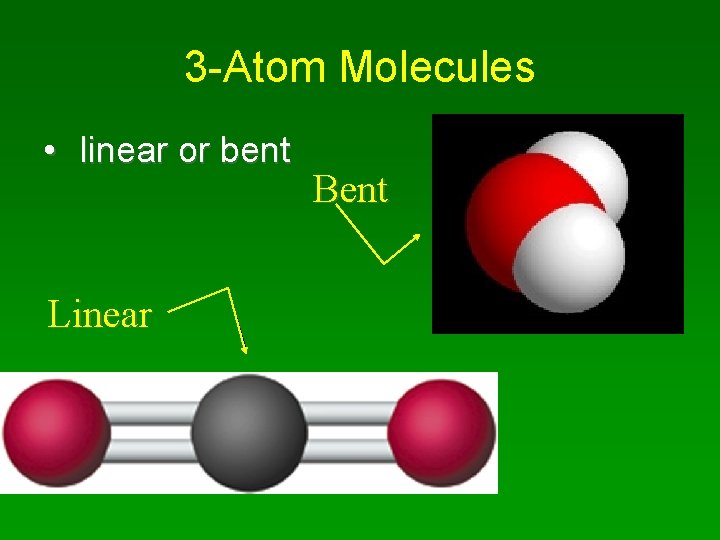
3 -Atom Molecules • linear or bent Linear Bent
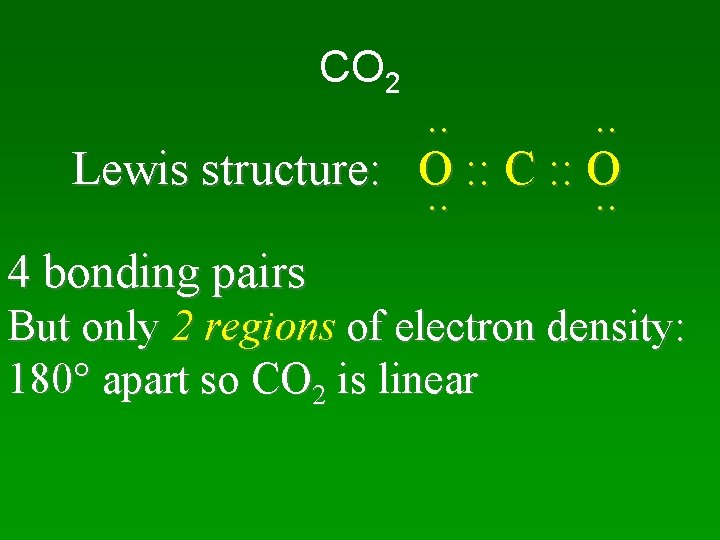
CO 2 . . Lewis structure: O : : C : : O. . 4 bonding pairs But only 2 regions of electron density: 180 apart so CO 2 is linear
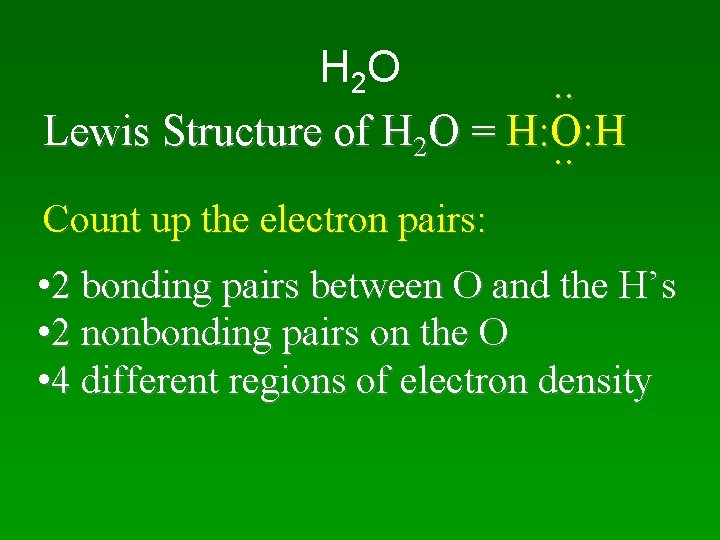
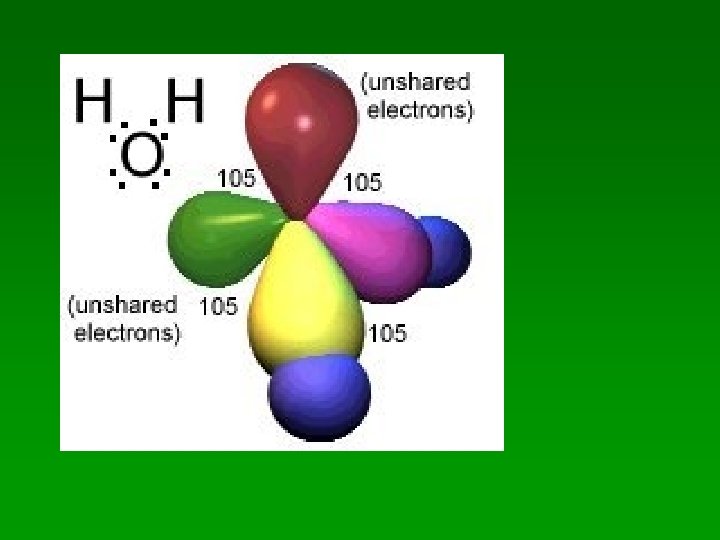
H 2 O. . Lewis Structure of H 2 O = H: O: H. . Count up the electron pairs: • 2 bonding pairs between O and the H’s • 2 nonbonding pairs on the O • 4 different regions of electron density
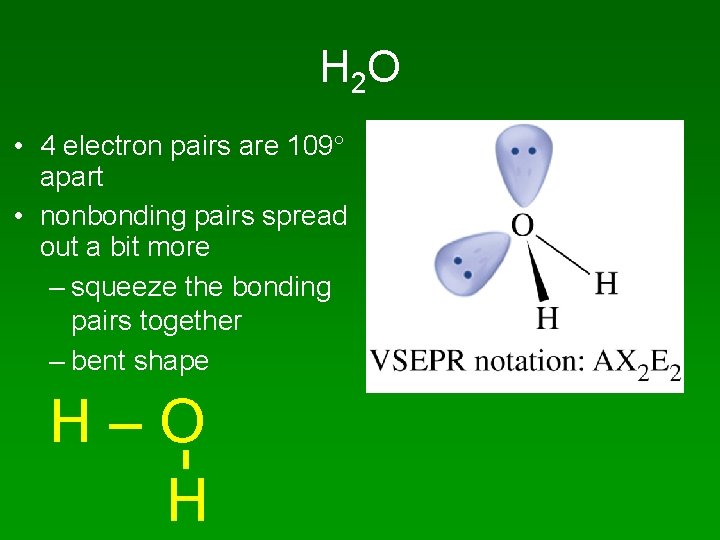
H 2 O • 4 electron pairs are 109 apart • nonbonding pairs spread out a bit more – squeeze the bonding pairs together – bent shape H–O H

3 -Atom Molecules • Triangular? Yes, ozone (O 3) is triangular

4 -Atom Molecules • Two possibilities: –Trigonal Planar – in 1 plane –Trigonal Pyramidal
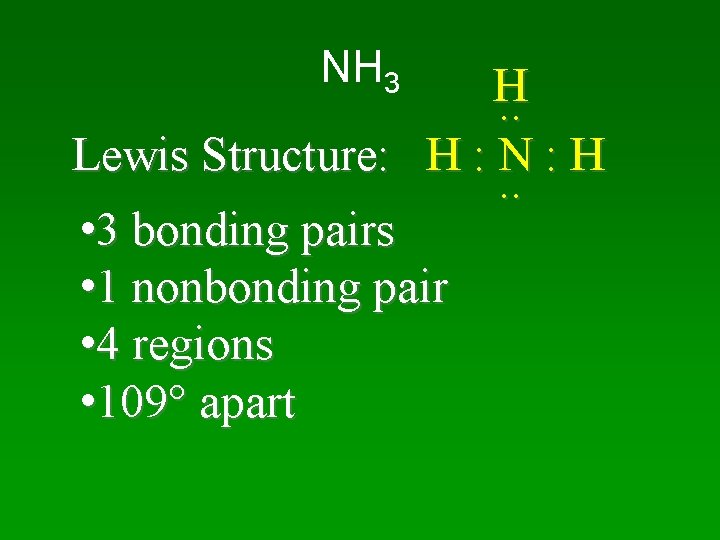
NH 3 H. . Lewis Structure: H : N : H. . • 3 bonding pairs • 1 nonbonding pair • 4 regions • 109 apart
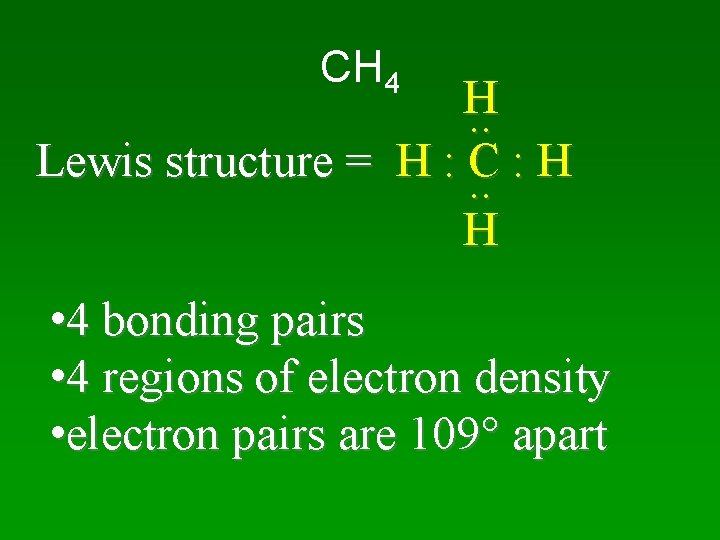
CH 4 H. . Lewis structure = H : C : H. . H • 4 bonding pairs • 4 regions of electron density • electron pairs are 109 apart
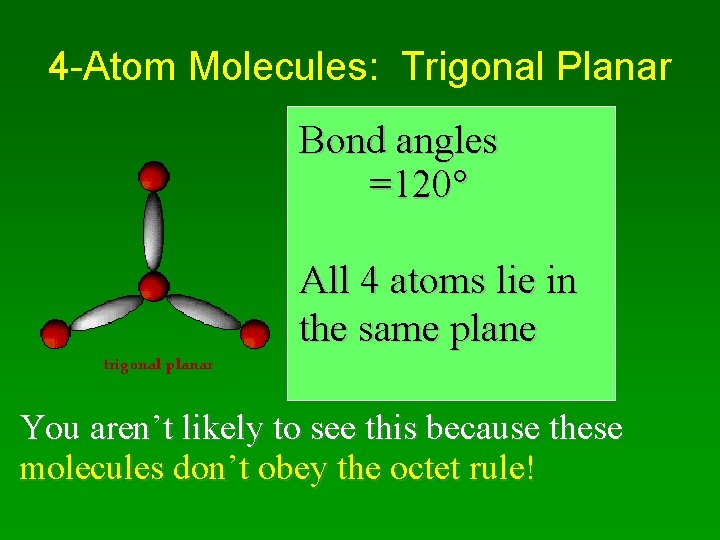
4 -Atom Molecules: Trigonal Planar Bond angles =120 All 4 atoms lie in the same plane You aren’t likely to see this because these molecules don’t obey the octet rule!
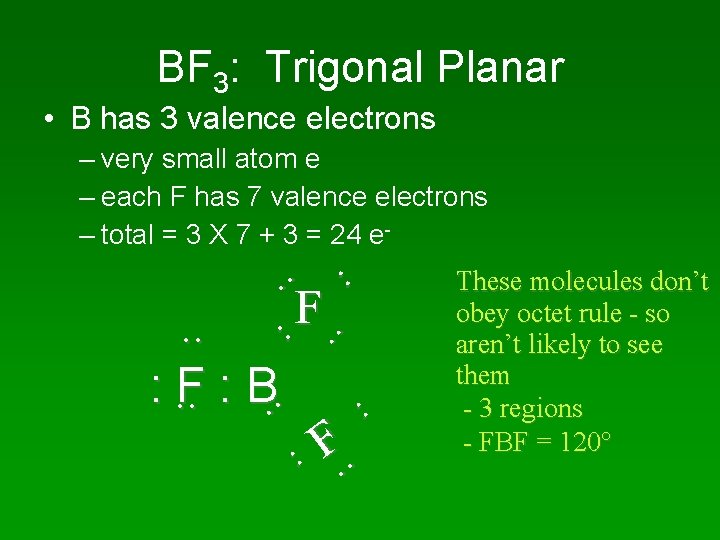
BF 3: Trigonal Planar • B has 3 valence electrons – very small atom e – each F has 7 valence electrons – total = 3 X 7 + 3 = 24 e- . . : . . F : B . . F . . F. . These molecules don’t obey octet rule - so aren’t likely to see them - 3 regions - FBF = 120
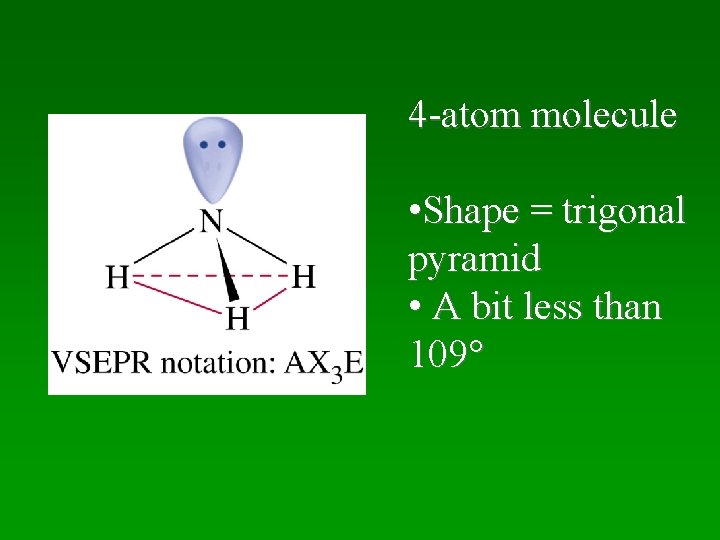
4 -atom molecule • Shape = trigonal pyramid • A bit less than 109
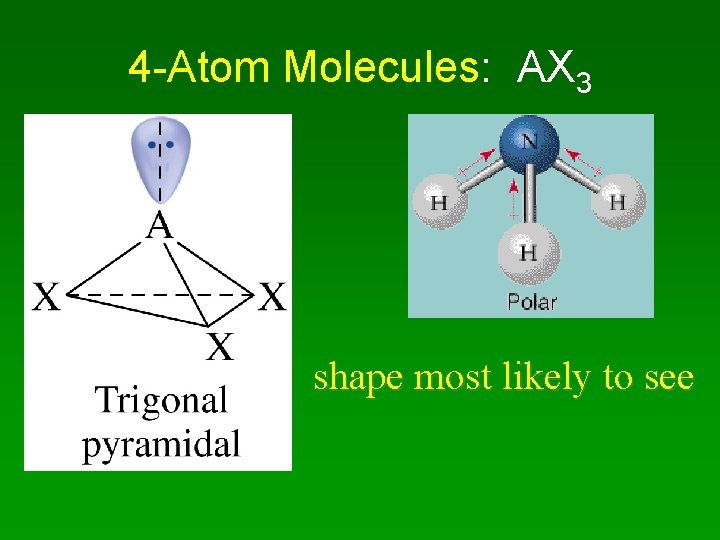
4 -Atom Molecules: AX 3 shape most likely to see

5 -Atom Molecules: AX 4
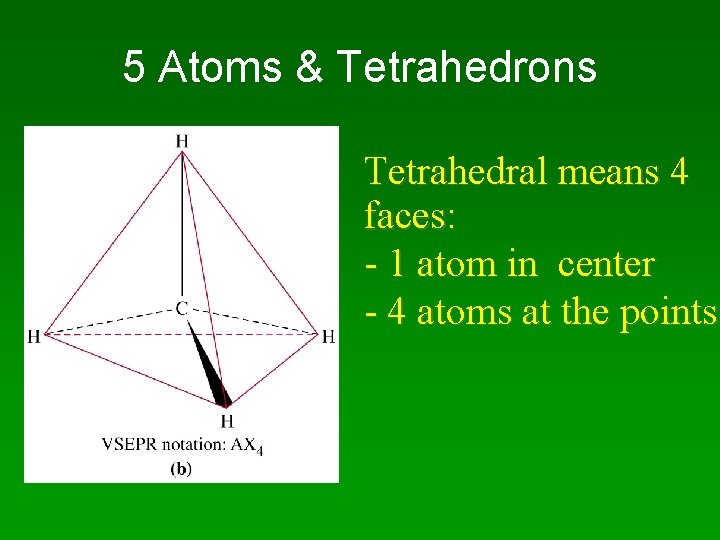
5 Atoms & Tetrahedrons Tetrahedral means 4 faces: - 1 atom in center - 4 atoms at the points
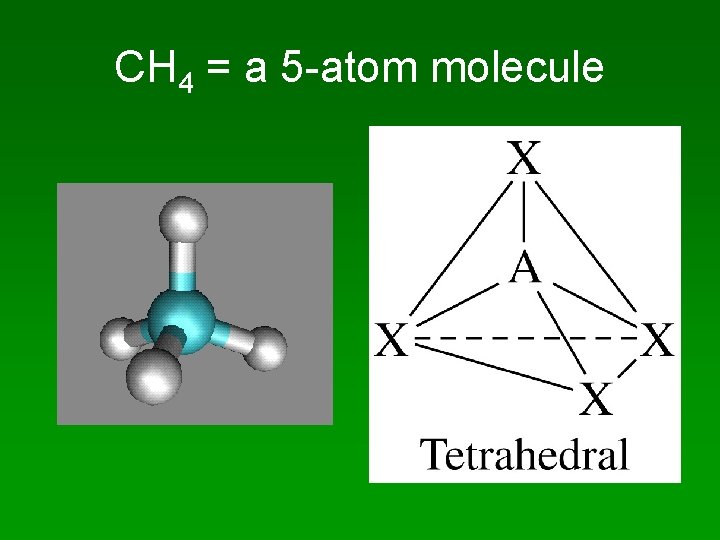
CH 4 = a 5 -atom molecule

CH 4 = a 5 -atom molecule
Summary of Molecular Shapes • Start with Lewis Structure! • Look at # regions of electron density on central atom • Look at # atoms bonded to central atom
Molecular Polarity • Look at type bonds in molecule • Look at shape of molecule • nonpolar molecular must be symmetrical
Molecular Polarity • molecule is symmetric if: – electrical charge on 1 side = electrical charge at matching point on opposite side – “pull” of one polar bond is offset by “pull” of another polar bond – tug-of-war that can't be won!
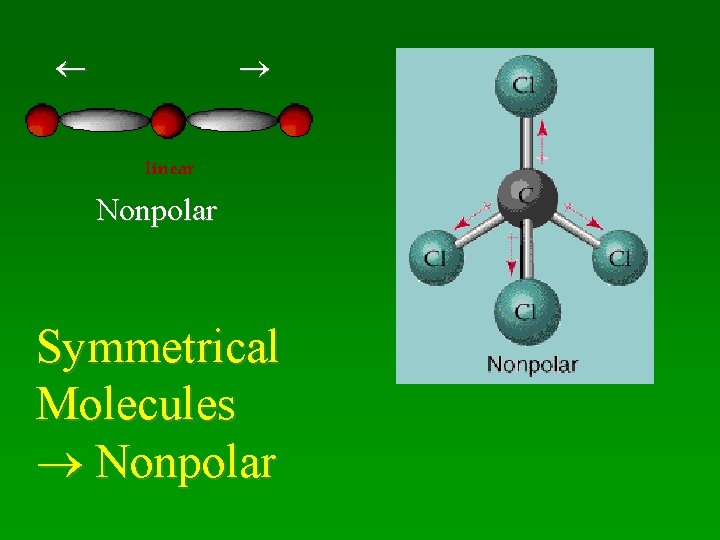
Nonpolar Symmetrical Molecules Nonpolar
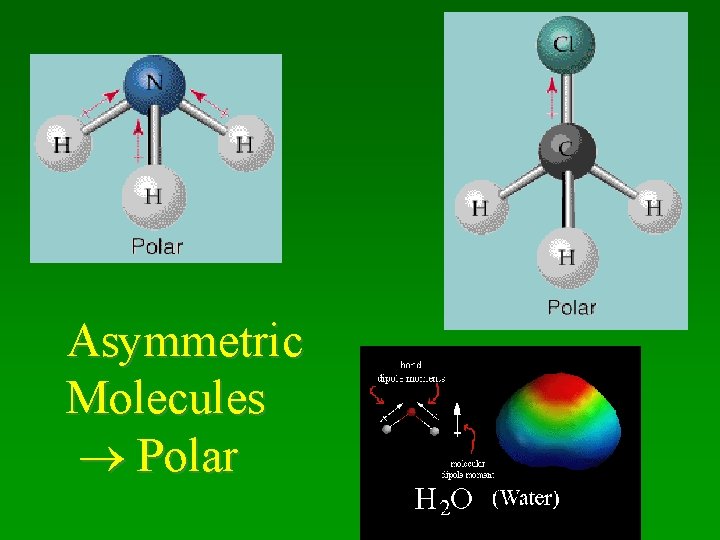
Asymmetric Molecules Polar
- Slides: 30