Molecular Shape and Polarity Intermolecular Forces of Attraction









- Slides: 46

Molecular Shape and Polarity Intermolecular Forces of Attraction States of Matter VSEPR Theory

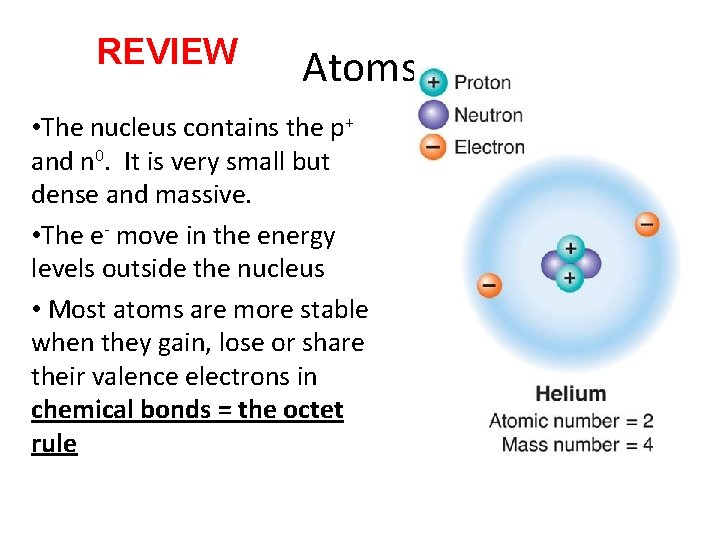
REVIEW Atoms • The nucleus contains the p+ and n 0. It is very small but dense and massive. • The e- move in the energy levels outside the nucleus • Most atoms are more stable when they gain, lose or share their valence electrons in chemical bonds = the octet rule

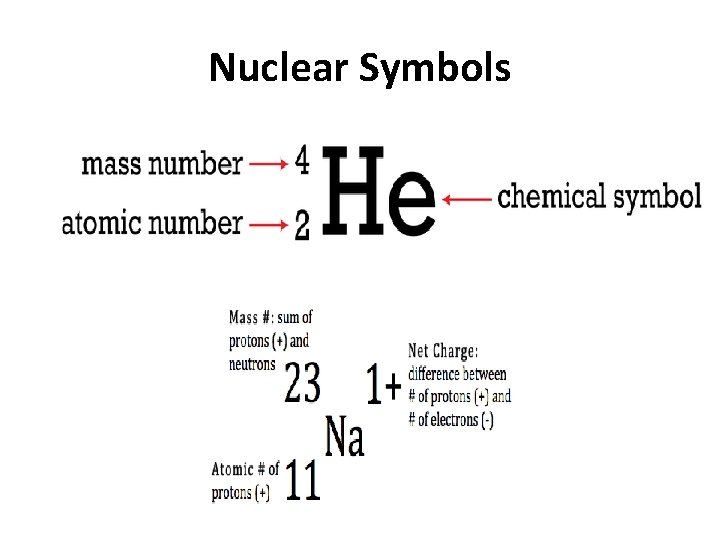
Nuclear Symbols

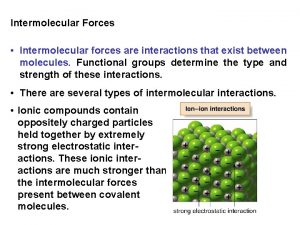
Ionic Bonds make crystalline solids – Form when e- are transferred from one atom to another as they try to complete their outer energy level. – Metal Atom loses e-: gets a positive charge. – Nonmetal Atom gains e-: gets a negative charge. – Positively and negatively charged atoms (or groups of atoms) are known as ions. – Think rocks, salts and minerals in earth’s crust.

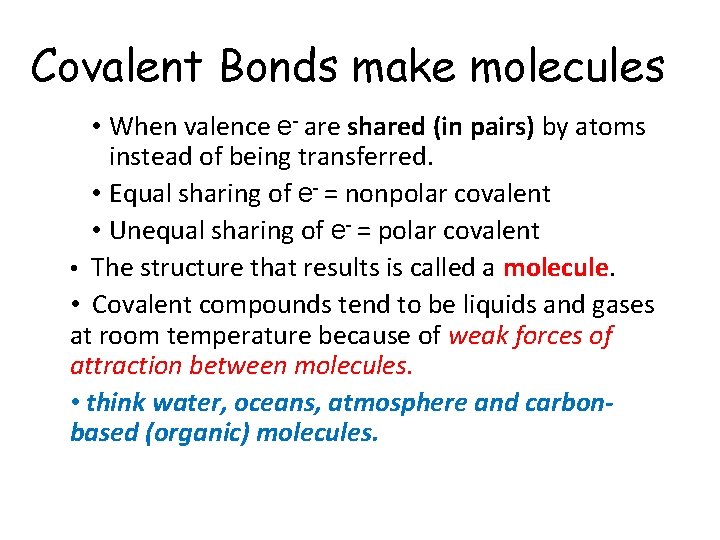
Covalent Bonds make molecules • When valence e- are shared (in pairs) by atoms instead of being transferred. • Equal sharing of e- = nonpolar covalent • Unequal sharing of e- = polar covalent • The structure that results is called a molecule. • Covalent compounds tend to be liquids and gases at room temperature because of weak forces of attraction between molecules. • think water, oceans, atmosphere and carbonbased (organic) molecules.

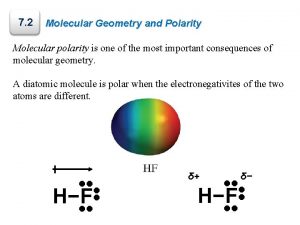
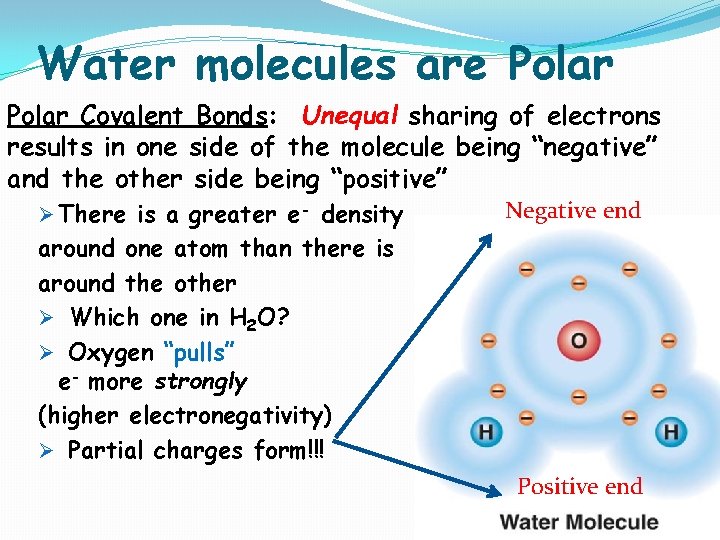
Water molecules are Polar Covalent Bonds: Unequal sharing of electrons results in one side of the molecule being “negative” and the other side being “positive” Ø There is a greater e- density Negative end around one atom than there is around the other Ø Which one in H 2 O? Ø Oxygen “pulls” e- more strongly (higher electronegativity) Ø Partial charges form!!! Positive end

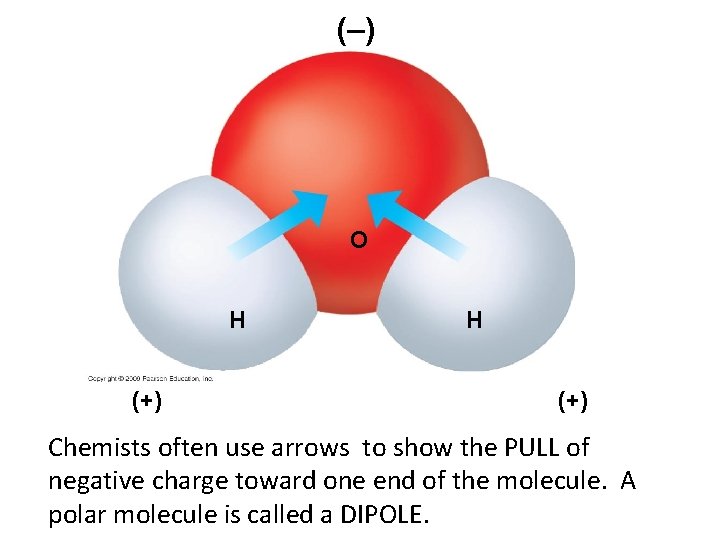
(–) O H (+) Chemists often use arrows to show the PULL of negative charge toward one end of the molecule. A polar molecule is called a DIPOLE.

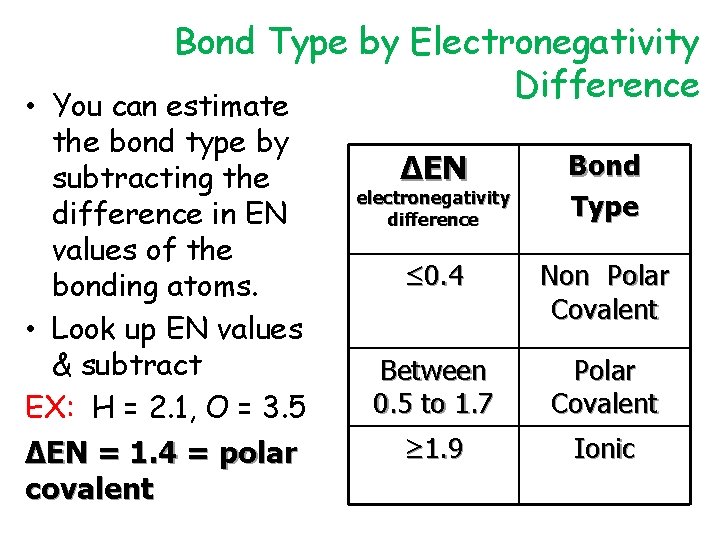
Bond Type by Electronegativity Difference • You can estimate the bond type by subtracting the difference in EN values of the bonding atoms. • Look up EN values & subtract EX: H = 2. 1, O = 3. 5 ∆EN = 1. 4 = polar covalent ∆EN Bond Type ≤ 0. 4 Non Polar Covalent Between 0. 5 to 1. 7 Polar Covalent ≥ 1. 9 Ionic electronegativity difference

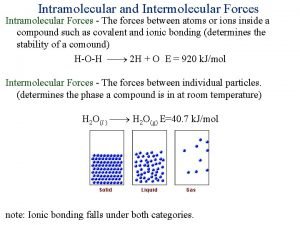
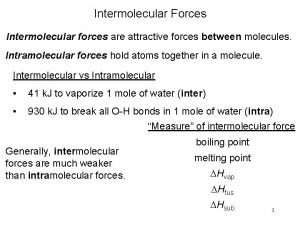
Ionic vs Molecular & States of Matter • Much of chemistry comes down to opposite charges attract and like charges repel. • This means that PROPERTIES like physical state and melting and boiling points DEPEND ON HOW STRONGLY THE BASIC UNITS ARE ATTRACTED TO EACH OTHER! • Solids melt and liquids boil when the attractive forces between the particles are broken…. Ø Stronger attractive forces result in higher MPts and BPts.

Ionic vs Molecular & States of Matter • The force of attraction between ions is quite strong – ionic crystals are hard, brittle solids with high MPts – it takes more energy to break these bonds. • The force of attraction between individual molecules are called weak forces – many molecular substances are gases or volatile liquids at room temp. and MPts are low.

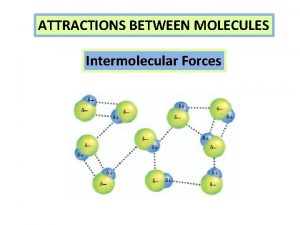
INTERMOLECULAR FORCES: Weak attractive forces between molecules • Chemists call intermolecular forces of attraction Van der Waals forces, after the scientist who discovered them. • IM forces vary in strength and change the properties of molecular substances. • IM forces are due to polarity in molecules – differences depend on whether dipoles are permanent or temporary. • IM forces allow molecules to come together and form liquids, like water, and a few covalent solids.

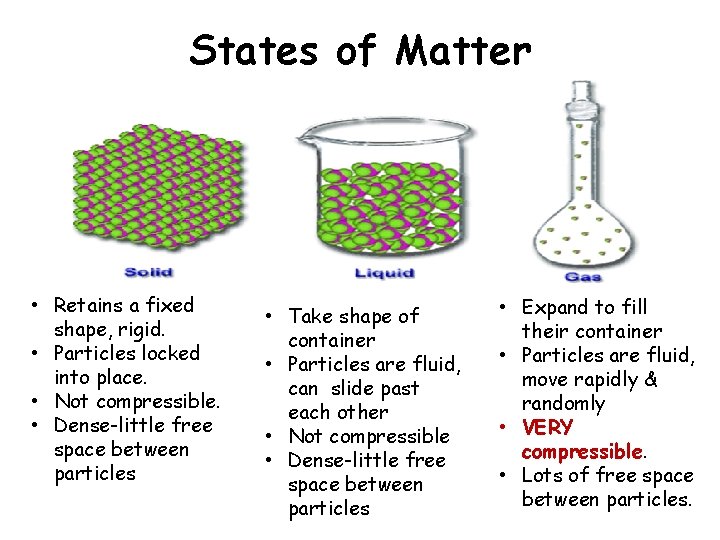
States of Matter • Retains a fixed shape, rigid. • Particles locked into place. • Not compressible. • Dense-little free space between particles • Take shape of container • Particles are fluid, can slide past each other • Not compressible • Dense-little free space between particles • Expand to fill their container • Particles are fluid, move rapidly & randomly • VERY compressible. • Lots of free space between particles.

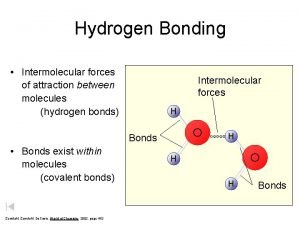
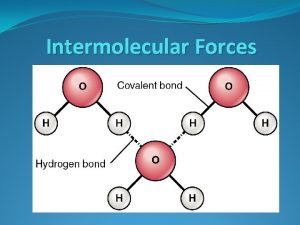
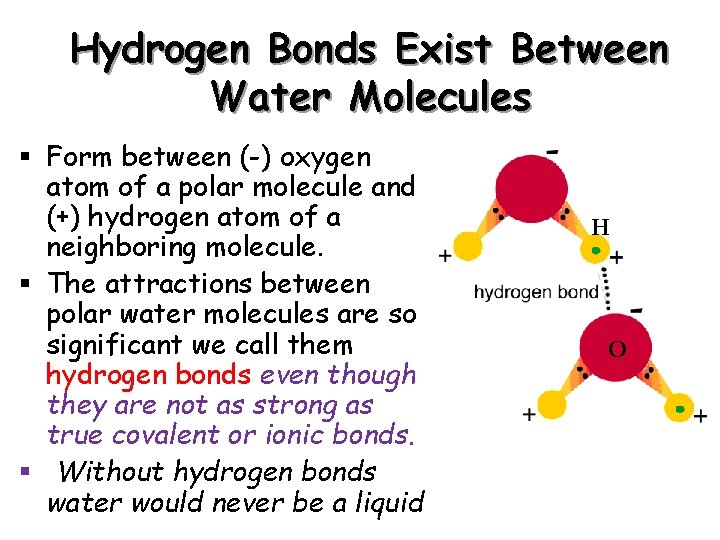
Hydrogen Bonds Exist Between Water Molecules § Form between (-) oxygen atom of a polar molecule and (+) hydrogen atom of a neighboring molecule. § The attractions between polar water molecules are so significant we call them hydrogen bonds even though they are not as strong as true covalent or ionic bonds. § Without hydrogen bonds water would never be a liquid H O

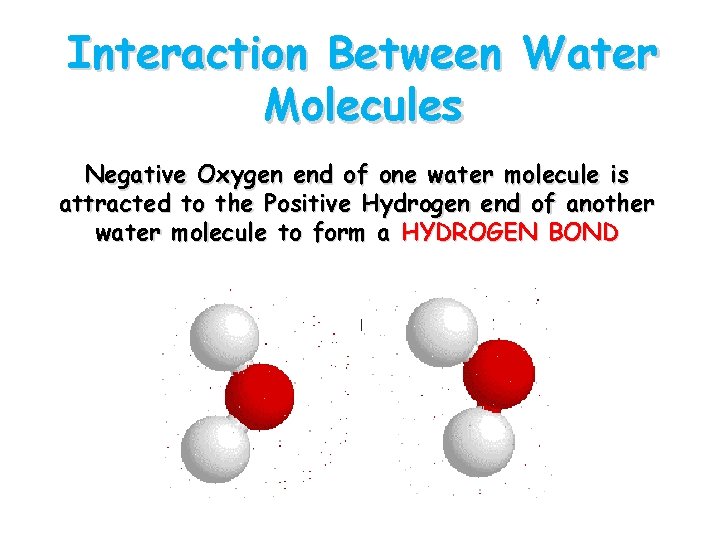
Interaction Between Water Molecules Negative Oxygen end of one water molecule is attracted to the Positive Hydrogen end of another water molecule to form a HYDROGEN BOND

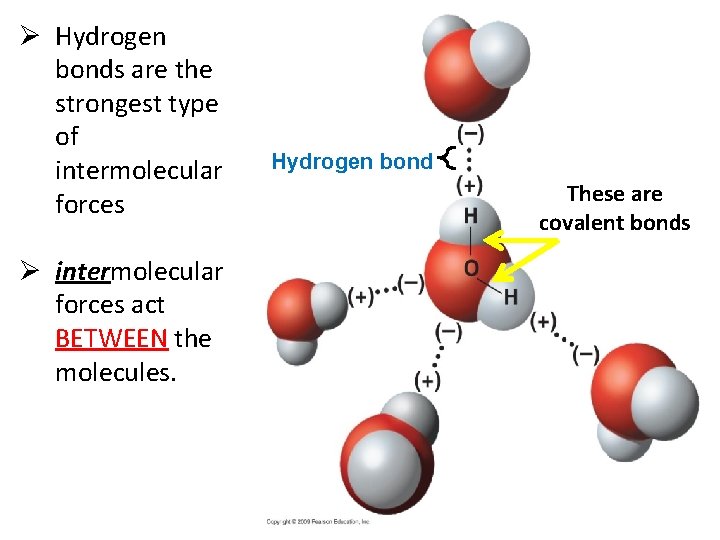
Ø Hydrogen bonds are the strongest type of intermolecular forces Ø intermolecular forces act BETWEEN the molecules. Hydrogen bond These are covalent bonds

Hydrogen Bonds – Why we are here… • Hydrogen bonds make water a liquid at room temperature • Hydrogen bonds allow DNA to unzip during replication • White = H and Red = O

Video: Geckos • https: //youtu. be/Ye. Su. Qm 7 Kfa. E

Video: Van der Waals Forces • https: //youtu. be/3 y. Xr. Hl. LZ 4 LI

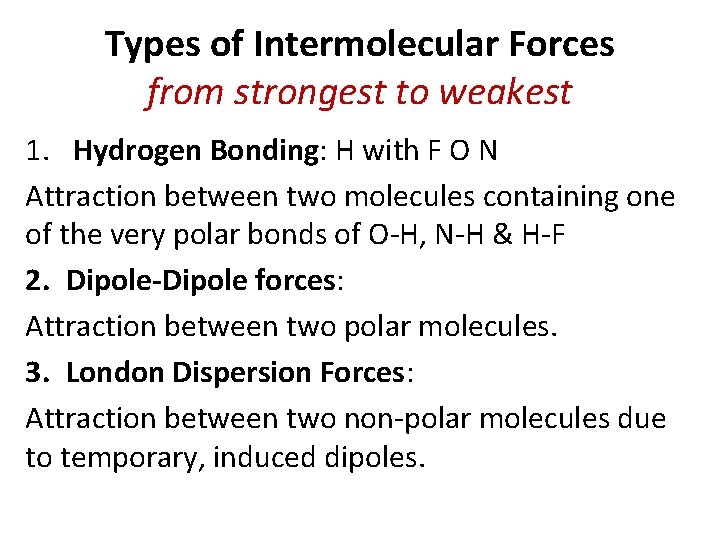
Types of Intermolecular Forces from strongest to weakest 1. Hydrogen Bonding: H with F O N Attraction between two molecules containing one of the very polar bonds of O-H, N-H & H-F 2. Dipole-Dipole forces: Attraction between two polar molecules. 3. London Dispersion Forces: Attraction between two non-polar molecules due to temporary, induced dipoles.

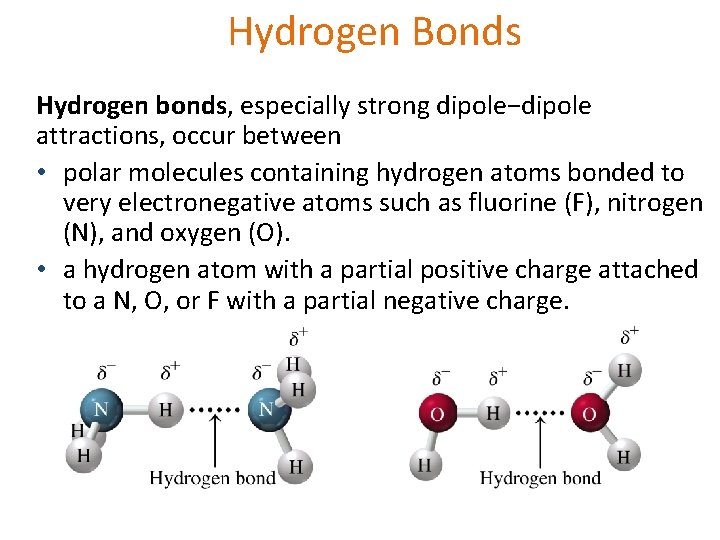
Hydrogen Bonds Hydrogen bonds, especially strong dipole−dipole attractions, occur between • polar molecules containing hydrogen atoms bonded to very electronegative atoms such as fluorine (F), nitrogen (N), and oxygen (O). • a hydrogen atom with a partial positive charge attached to a N, O, or F with a partial negative charge.

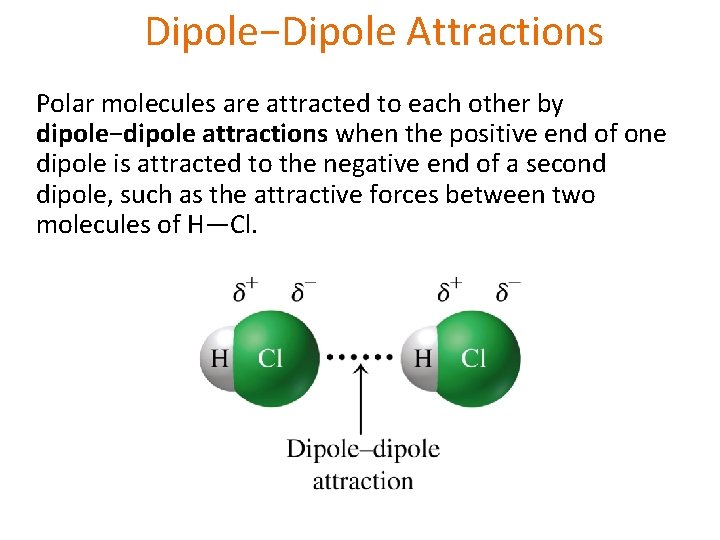
Dipole−Dipole Attractions Polar molecules are attracted to each other by dipole−dipole attractions when the positive end of one dipole is attracted to the negative end of a second dipole, such as the attractive forces between two molecules of H—Cl.

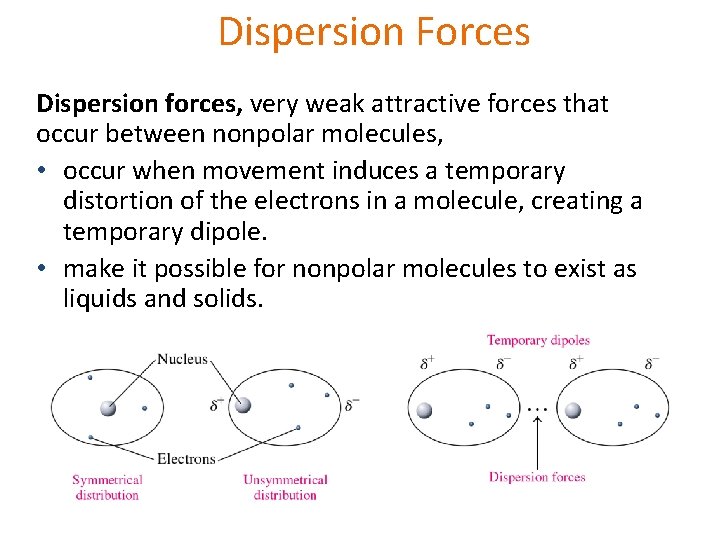
Dispersion Forces Dispersion forces, very weak attractive forces that occur between nonpolar molecules, • occur when movement induces a temporary distortion of the electrons in a molecule, creating a temporary dipole. • make it possible for nonpolar molecules to exist as liquids and solids.

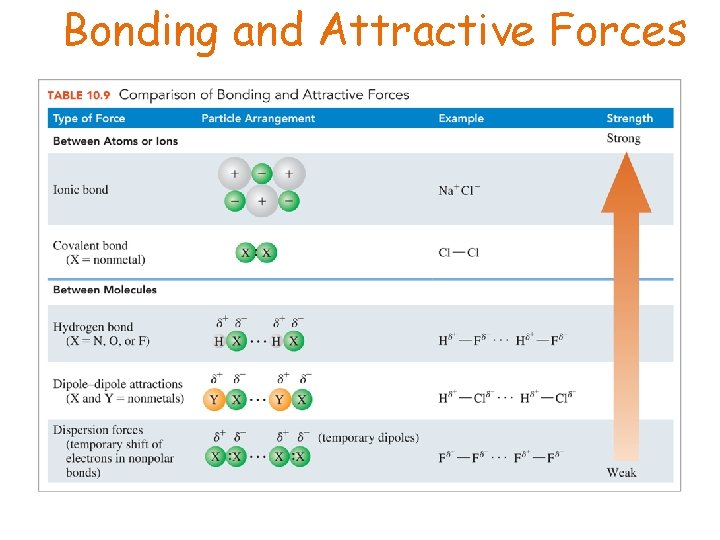
Bonding and Attractive Forces

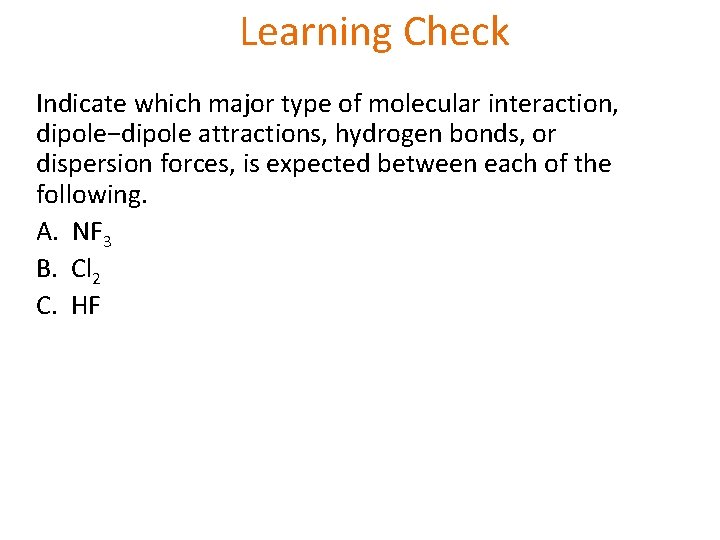
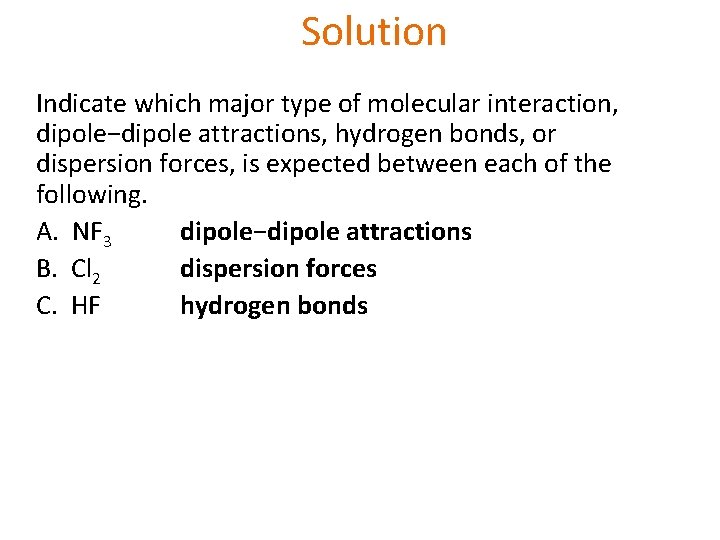
Learning Check Indicate which major type of molecular interaction, dipole−dipole attractions, hydrogen bonds, or dispersion forces, is expected between each of the following. A. NF 3 B. Cl 2 C. HF

Solution Indicate which major type of molecular interaction, dipole−dipole attractions, hydrogen bonds, or dispersion forces, is expected between each of the following. A. NF 3 dipole−dipole attractions B. Cl 2 dispersion forces C. HF hydrogen bonds

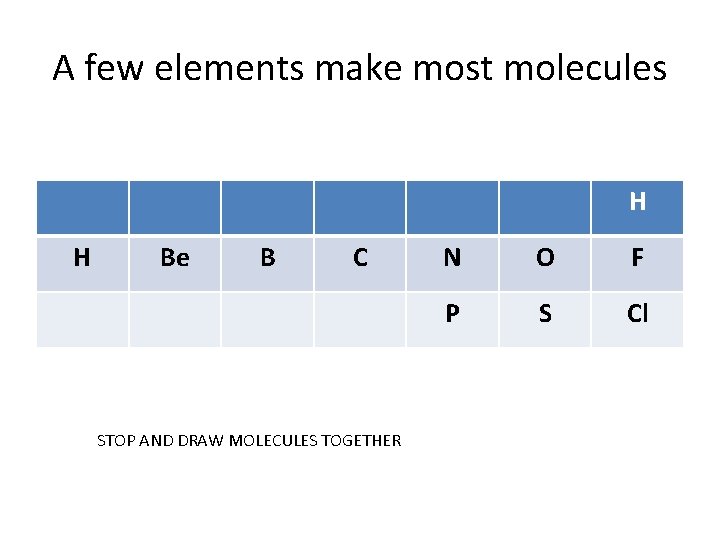
A few elements make most molecules H H Be B C STOP AND DRAW MOLECULES TOGETHER N O F P S Cl

VSEPR Theory • Valence Shell Electron Pair Repulsion Theory • States that: Repulsion between sets of valence electrons surrounding an atom causes them to be oriented as far apart as possible. VSEPR theory postulates that the lone pairs occupy space around the central atom just like bonding pairs, but they repel other electron pairs more strongly than bonding pairs do.

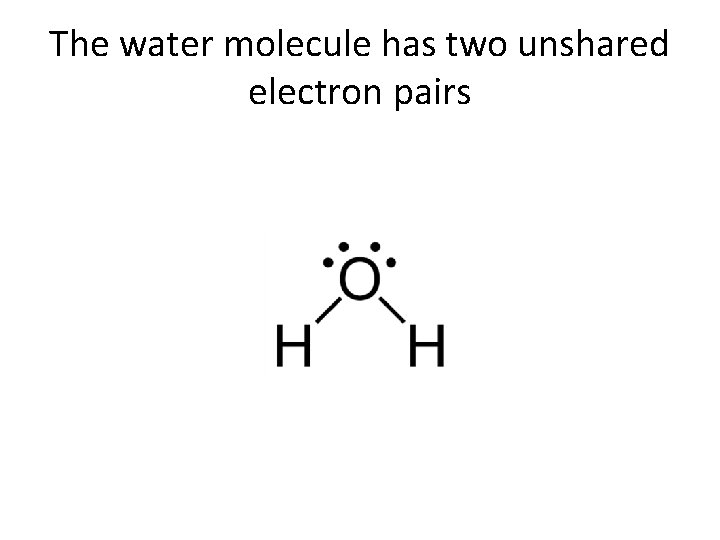
The water molecule has two unshared electron pairs

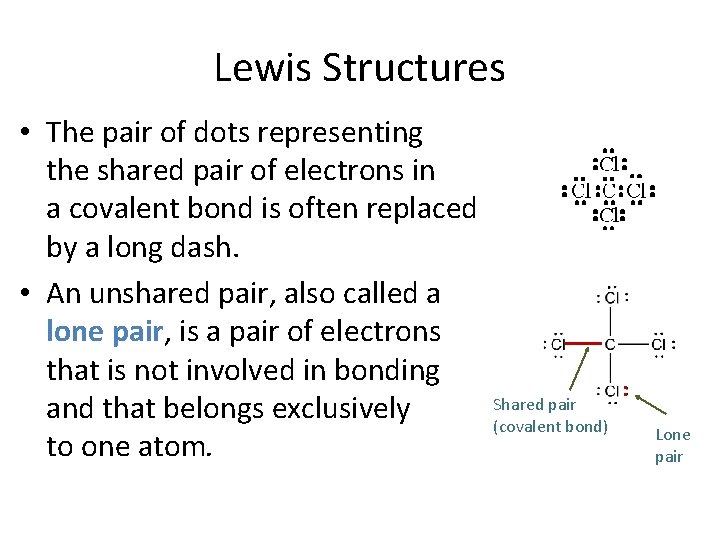
Lewis Structures • The pair of dots representing the shared pair of electrons in a covalent bond is often replaced by a long dash. • An unshared pair, also called a lone pair, is a pair of electrons that is not involved in bonding and that belongs exclusively to one atom. Shared pair (covalent bond) • • Lone pair

The five most common shapes of small molecules 1. 2. 3. 4. 5. Linear Trigonal Planar Tetrahedral Trigonal Pyramidal Bent

Molecular Geometry • The shape of molecules affects the physical & chemical properties of molecular (covalent) substances. • All molecules have a symmetrical shape because the bonds & atoms are arranged with equal distances separating atoms that are NOT bonded to each other. • WHY? A repulsive force exists between e- pairs in molecules.

How do molecules get their shapes? • Valence e- surrounding an atom may be shared in pairs OR left unshared. • Both bonded and unbonded e- pairs will repel each other – LIKE CHARGES REPEL. • Unshared e- pairs repel each other VERY STRONGLY!!!

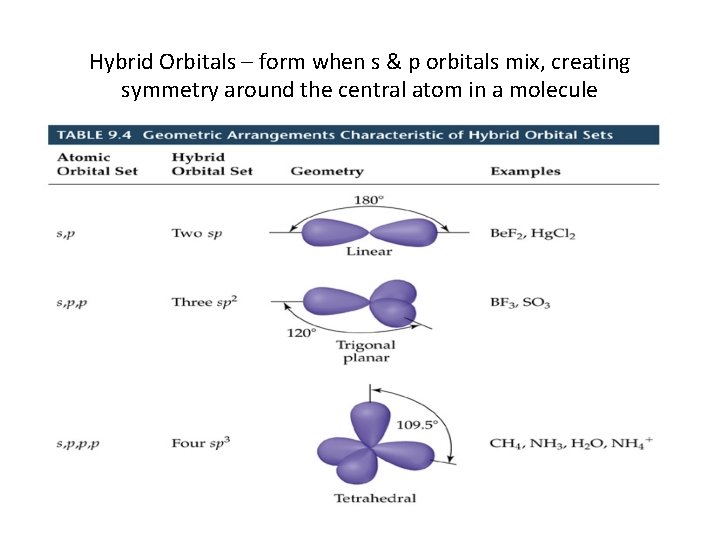
Hybrid Orbitals – form when s & p orbitals mix, creating symmetry around the central atom in a molecule

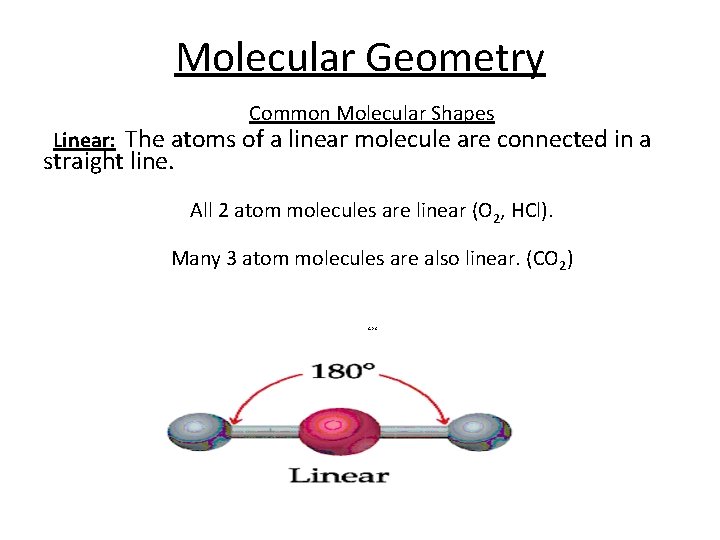
Molecular Geometry Common Molecular Shapes Linear: The atoms of a linear molecule are connected in a straight line. All 2 atom molecules are linear (O 2, HCl). Many 3 atom molecules are also linear. (CO 2) 6 -26

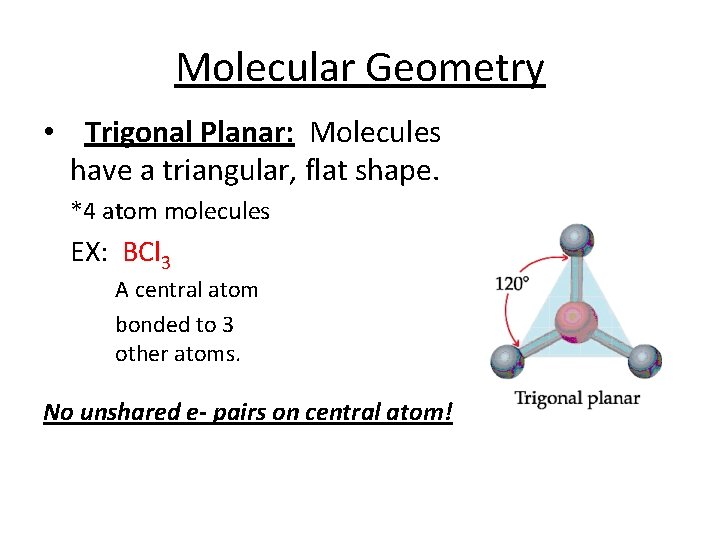
Molecular Geometry • Trigonal Planar: Molecules have a triangular, flat shape. *4 atom molecules EX: BCl 3 A central atom bonded to 3 other atoms. No unshared e- pairs on central atom!

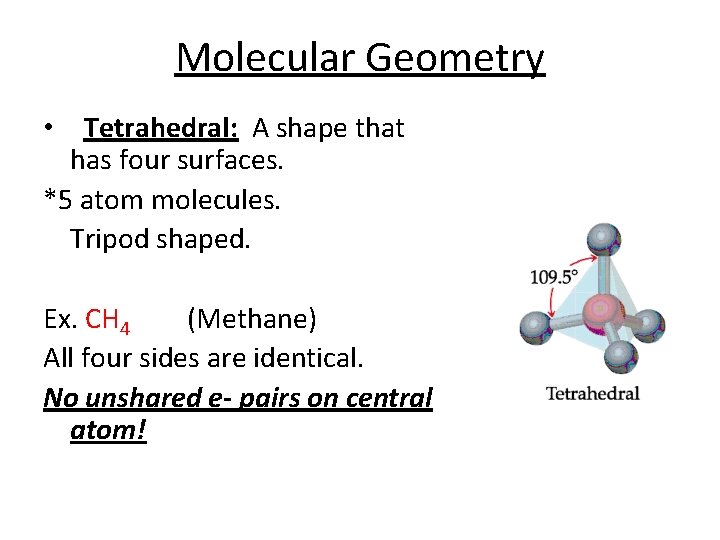
Molecular Geometry • Tetrahedral: A shape that has four surfaces. *5 atom molecules. Tripod shaped. Ex. CH 4 (Methane) All four sides are identical. No unshared e- pairs on central atom!

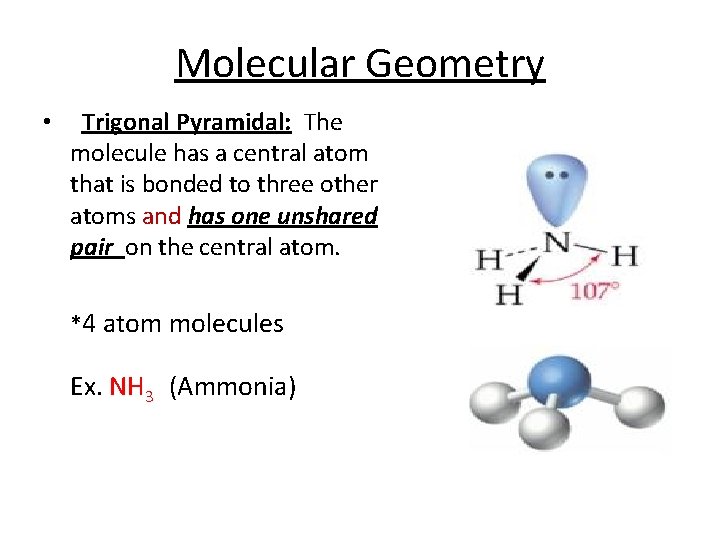
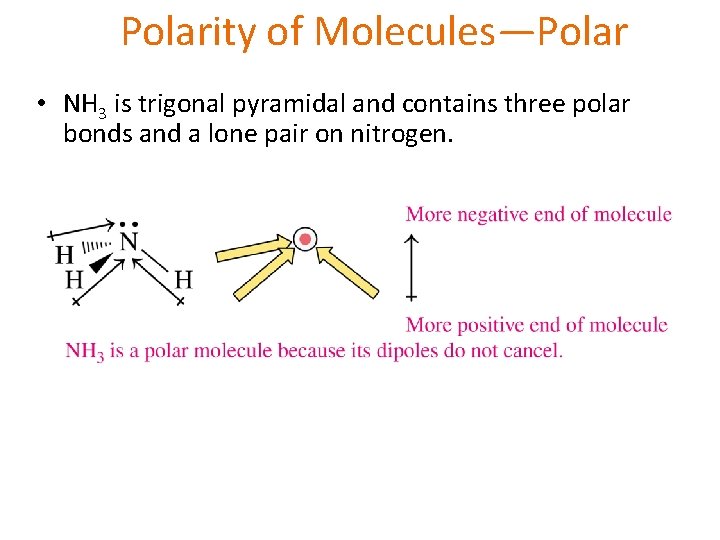
Molecular Geometry • Trigonal Pyramidal: The molecule has a central atom that is bonded to three other atoms and has one unshared pair on the central atom. *4 atom molecules Ex. NH 3 (Ammonia)

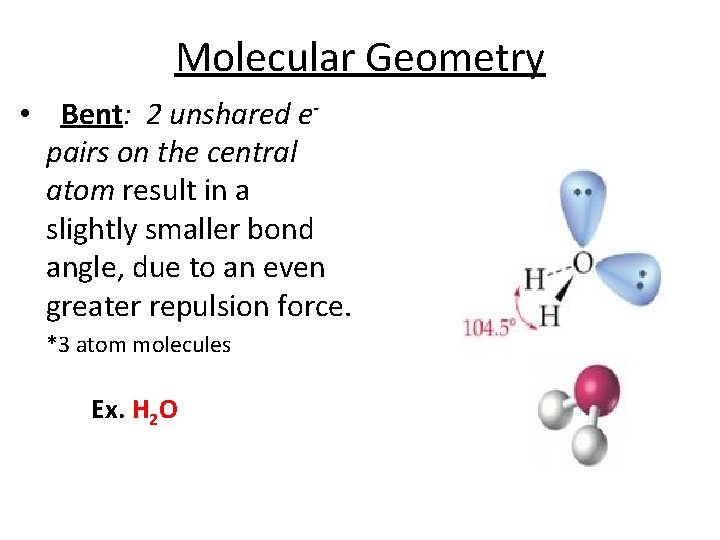
Molecular Geometry • Bent: 2 unshared epairs on the central atom result in a slightly smaller bond angle, due to an even greater repulsion force. *3 atom molecules Ex. H 2 O

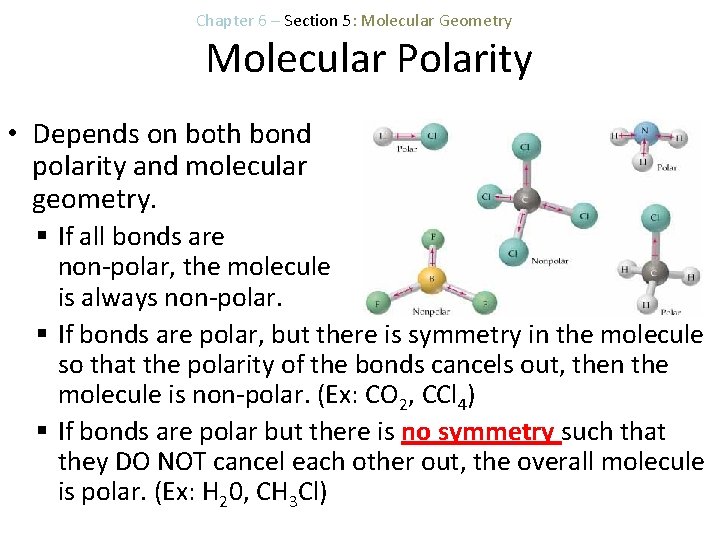
Chapter 6 – Section 5: Molecular Geometry Molecular Polarity • Depends on both bond polarity and molecular geometry. § If all bonds are non-polar, the molecule is always non-polar. § If bonds are polar, but there is symmetry in the molecule so that the polarity of the bonds cancels out, then the molecule is non-polar. (Ex: CO 2, CCl 4) § If bonds are polar but there is no symmetry such that they DO NOT cancel each other out, the overall molecule is polar. (Ex: H 20, CH 3 Cl)

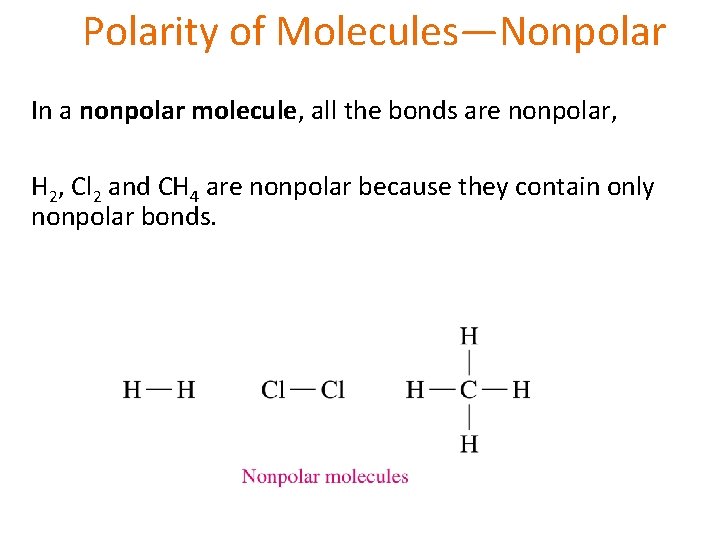
Polarity of Molecules—Nonpolar In a nonpolar molecule, all the bonds are nonpolar, H 2, Cl 2 and CH 4 are nonpolar because they contain only nonpolar bonds.

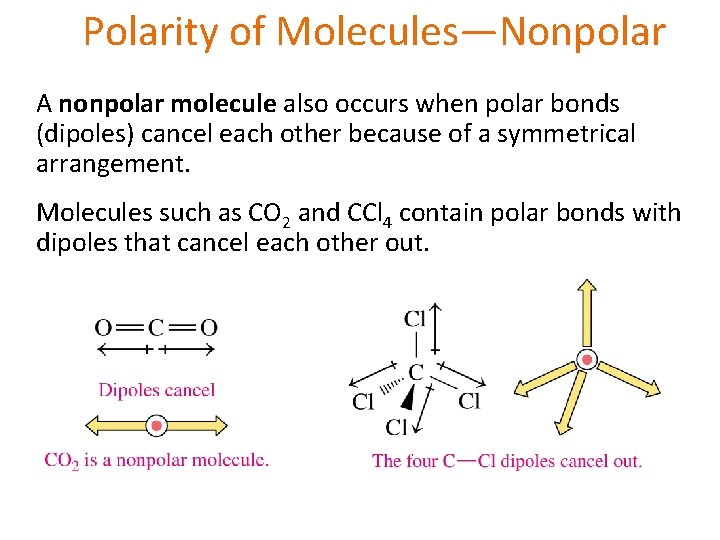
Polarity of Molecules—Nonpolar A nonpolar molecule also occurs when polar bonds (dipoles) cancel each other because of a symmetrical arrangement. Molecules such as CO 2 and CCl 4 contain polar bonds with dipoles that cancel each other out.

Polarity of Molecules—Polar A polar molecule occurs when the dipoles from individual bonds do not cancel each other out. For molecules with two or more electron groups, the shape (such as bent or trigonal pyramidal) determines whether or not the dipoles cancel.

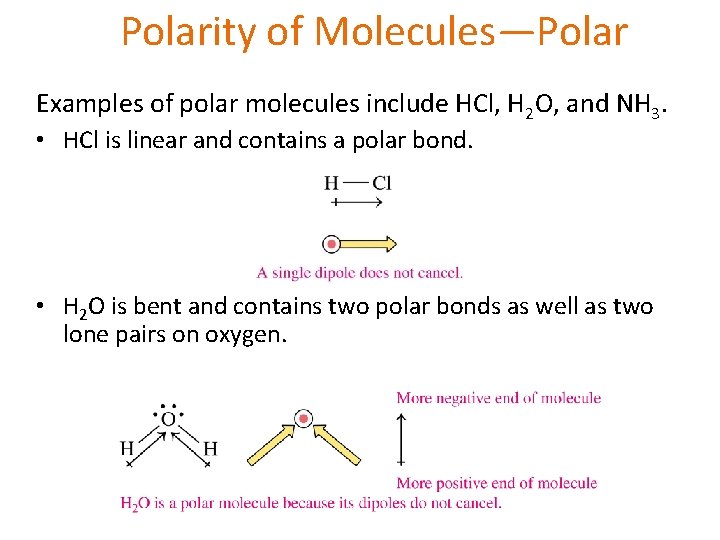
Polarity of Molecules—Polar Examples of polar molecules include HCl, H 2 O, and NH 3. • HCl is linear and contains a polar bond. • H 2 O is bent and contains two polar bonds as well as two lone pairs on oxygen.

Polarity of Molecules—Polar • NH 3 is trigonal pyramidal and contains three polar bonds and a lone pair on nitrogen.

Polarity of Molecules—Polar • CH 3 F is tetrahedral and contains three nonpolar bonds and a polar bond.

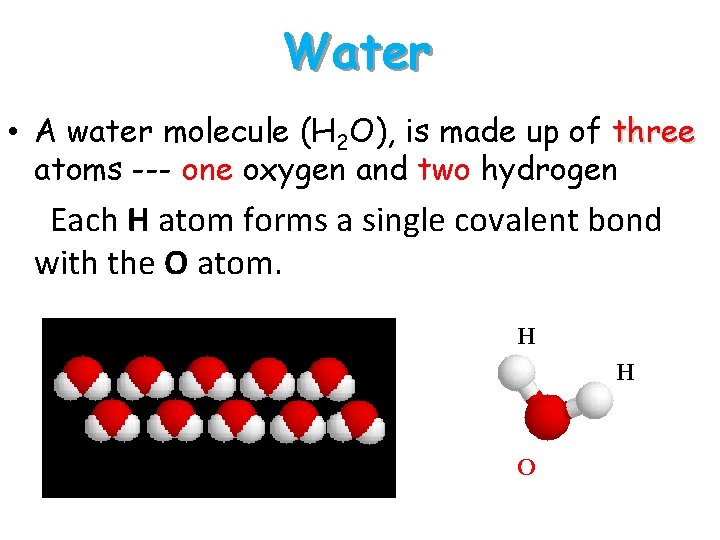
Water • A water molecule (H 2 O), is made up of three atoms --- one oxygen and two hydrogen. • Each H atom forms a single covalent bond with the O atom. H H H O White = H and Red = O H
 Nh3 shape and polarity
Nh3 shape and polarity Molecular polarity vs bond polarity
Molecular polarity vs bond polarity Intermolecular forces review
Intermolecular forces review Imfs
Imfs Hydrogen bonding as intermolecular forces
Hydrogen bonding as intermolecular forces Polarity worksheet
Polarity worksheet Intermolecular and intramolecular forces
Intermolecular and intramolecular forces Difference between intermolecular and intramolecular
Difference between intermolecular and intramolecular Inter vs intramolecular forces
Inter vs intramolecular forces Intramolecular forces
Intramolecular forces Intermolecular attraction
Intermolecular attraction Molecular shapes
Molecular shapes Molecular polarity
Molecular polarity Predicting molecular polarity
Predicting molecular polarity Molecular polarity definition
Molecular polarity definition Determining molecular polarity
Determining molecular polarity Molecular geometry polarity
Molecular geometry polarity Intermolecular and intramolecular forces
Intermolecular and intramolecular forces Geckos and intermolecular forces
Geckos and intermolecular forces Similarities of intermolecular and intramolecular forces
Similarities of intermolecular and intramolecular forces Viscosity and intermolecular forces
Viscosity and intermolecular forces Intermolecular forces capillary action
Intermolecular forces capillary action Intermolecular forces and boiling point
Intermolecular forces and boiling point Interatomic and intermolecular forces
Interatomic and intermolecular forces Unit 3 intermolecular forces and properties
Unit 3 intermolecular forces and properties London dispersion forces
London dispersion forces Electronegativity intermolecular forces
Electronegativity intermolecular forces Boiling point vs melting point
Boiling point vs melting point Imf
Imf Ionic forces of attraction
Ionic forces of attraction Mind map about friction
Mind map about friction London dispersion forces induced dipole
London dispersion forces induced dipole Webquest types of forces answers
Webquest types of forces answers Intermolecular forces
Intermolecular forces 3 types of intermolecular forces
3 types of intermolecular forces Imf chem
Imf chem Factors affecting solvation worksheet answers
Factors affecting solvation worksheet answers Dipole induced dipole interaction
Dipole induced dipole interaction Phthatic
Phthatic Dimethyl ether dipole dipole
Dimethyl ether dipole dipole Intermolecular forces symbol
Intermolecular forces symbol Strength of intermolecular forces
Strength of intermolecular forces Butanal intermolecular forces
Butanal intermolecular forces Strongest to weakest intermolecular forces
Strongest to weakest intermolecular forces Type of intermolecular forces
Type of intermolecular forces Ch2cl intermolecular forces
Ch2cl intermolecular forces Coh2 intermolecular forces
Coh2 intermolecular forces