MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING HOX






















- Slides: 41

MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES AND THE BODY PLAN

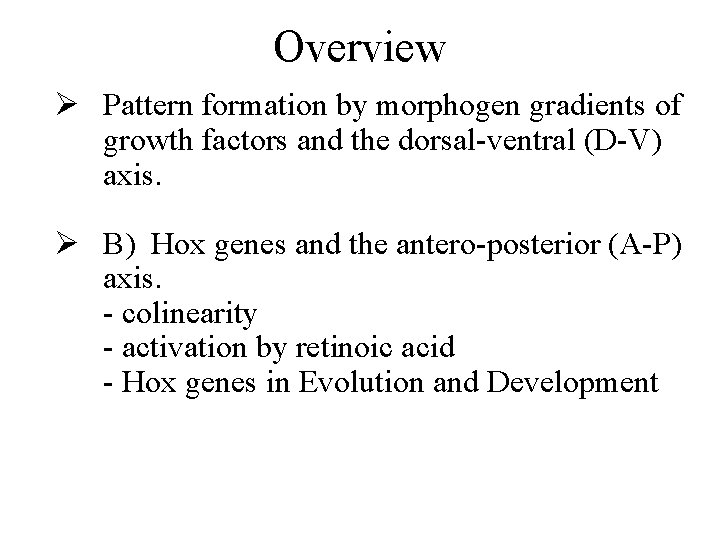
Overview Ø Pattern formation by morphogen gradients of growth factors and the dorsal-ventral (D-V) axis. Ø B) Hox genes and the antero-posterior (A-P) axis. - colinearity - activation by retinoic acid - Hox genes in Evolution and Development


During development groups of inducing cells called organizing centers secrete graded growth factor signals. The concentration gradient of a morphogen can induce multiple cell differentiation choices.

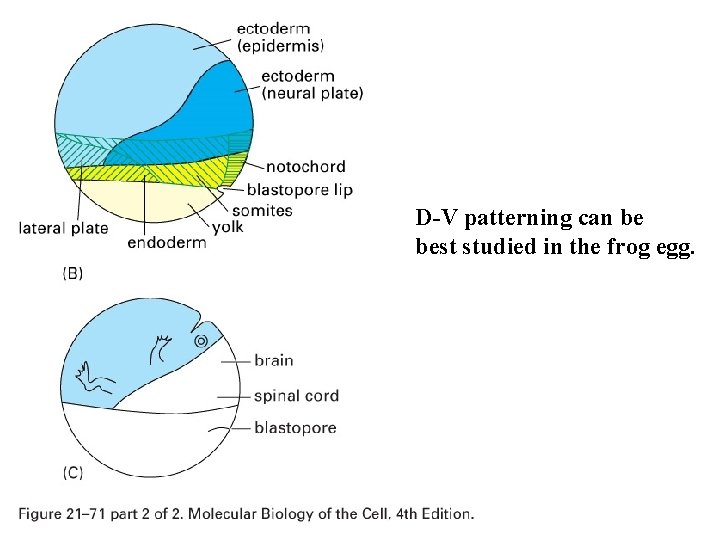
D-V patterning can be best studied in the frog egg.

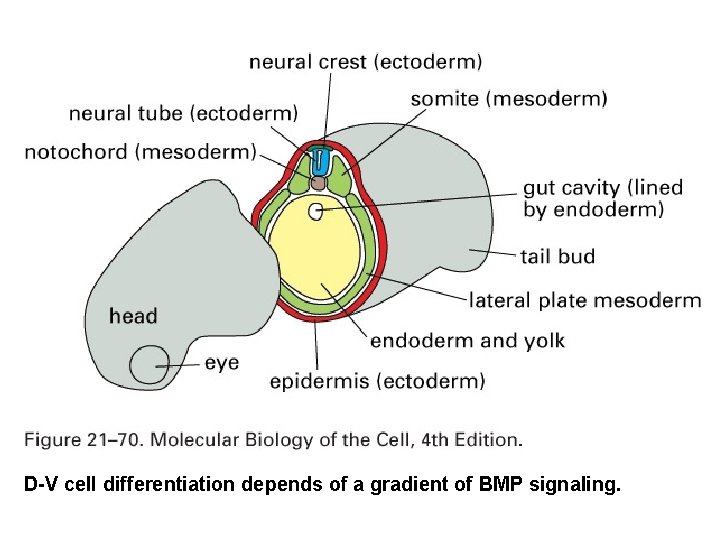
D-V cell differentiation depends of a gradient of BMP signaling.

Secreted antagonists such as Chordin and Noggin can bind to growth factors in the extracellular space and prevent binding to cell surface receptors. This inhibitory mechanism is used in development to generate morphogen gradients. Chordin establishes a BMP 4 gradient at gastrula. Chordin inhibits

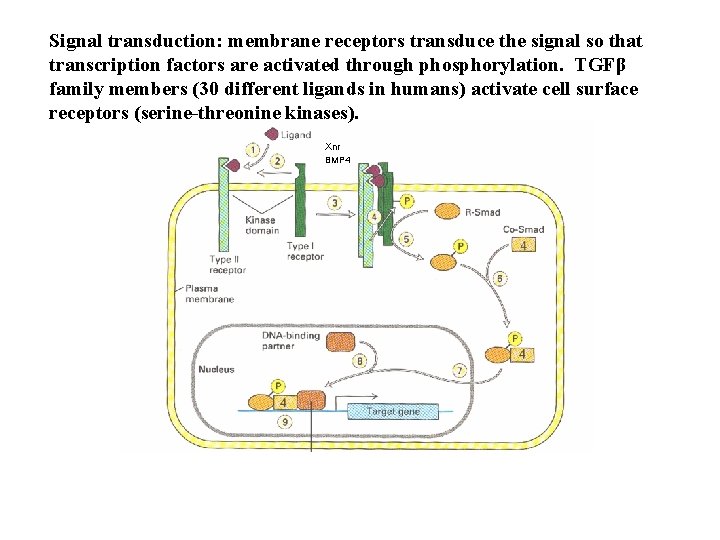
Signal transduction: membrane receptors transduce the signal so that transcription factors are activated through phosphorylation. TGFβ family members (30 different ligands in humans) activate cell surface receptors (serine-threonine kinases). Xnr BMP 4

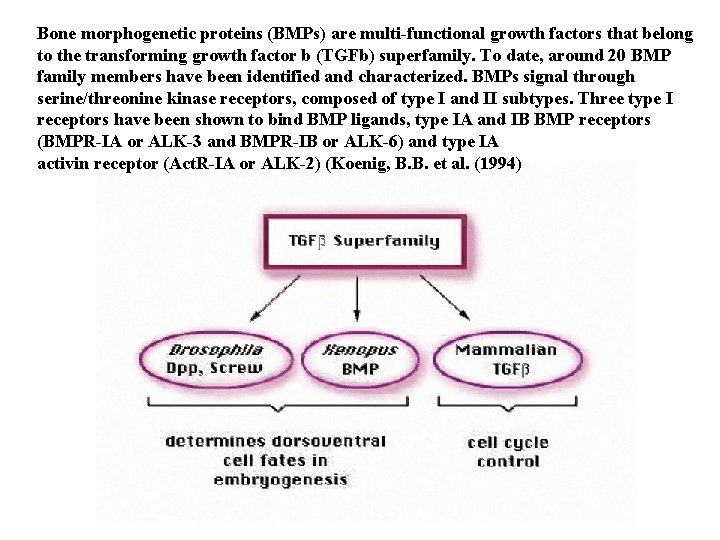
Bone morphogenetic proteins (BMPs) are multi-functional growth factors that belong to the transforming growth factor b (TGFb) superfamily. To date, around 20 BMP family members have been identified and characterized. BMPs signal through serine/threonine kinase receptors, composed of type I and II subtypes. Three type I receptors have been shown to bind BMP ligands, type IA and IB BMP receptors (BMPR-IA or ALK-3 and BMPR-IB or ALK-6) and type IA activin receptor (Act. R-IA or ALK-2) (Koenig, B. B. et al. (1994)

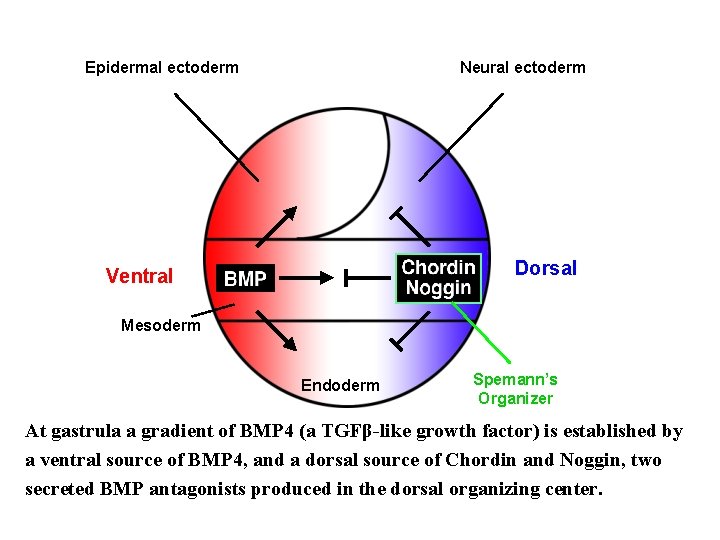
Epidermal ectoderm Neural ectoderm Dorsal Ventral Mesoderm Endoderm Spemann’s Organizer At gastrula a gradient of BMP 4 (a TGFβ-like growth factor) is established by a ventral source of BMP 4, and a dorsal source of Chordin and Noggin, two secreted BMP antagonists produced in the dorsal organizing center.


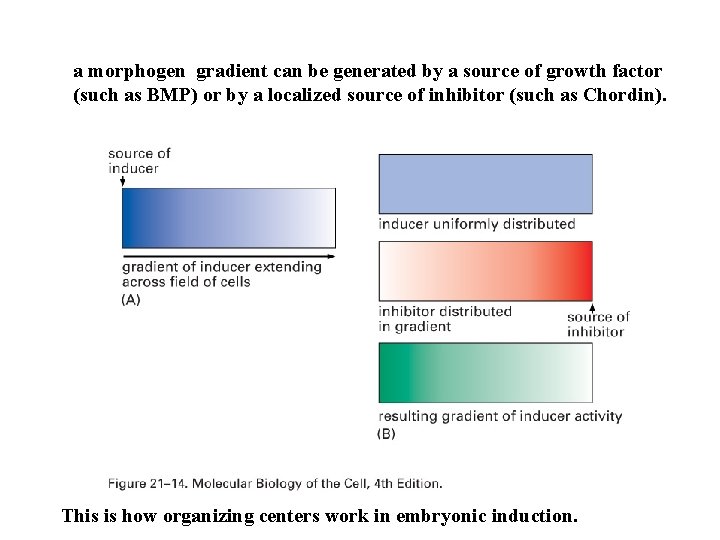
a morphogen gradient can be generated by a source of growth factor (such as BMP) or by a localized source of inhibitor (such as Chordin). This is how organizing centers work in embryonic induction.

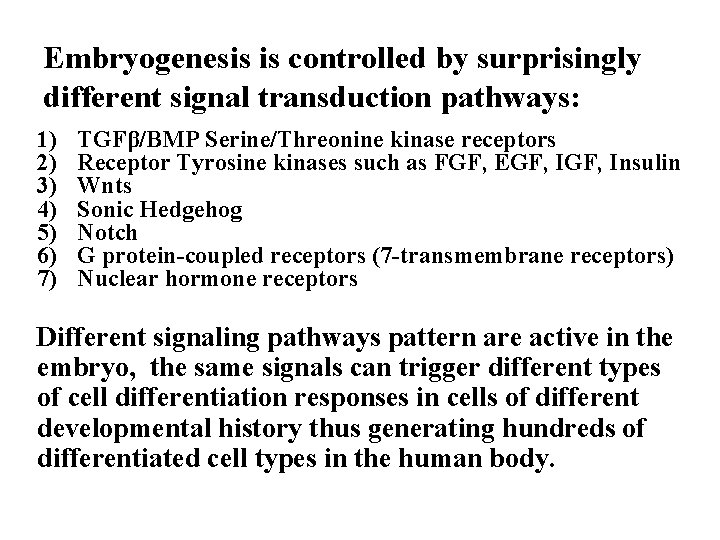
Embryogenesis is controlled by surprisingly different signal transduction pathways: 1) 2) 3) 4) 5) 6) 7) TGFβ/BMP Serine/Threonine kinase receptors Receptor Tyrosine kinases such as FGF, EGF, Insulin Wnts Sonic Hedgehog Notch G protein-coupled receptors (7 -transmembrane receptors) Nuclear hormone receptors Different signaling pathways pattern are active in the embryo, the same signals can trigger different types of cell differentiation responses in cells of different developmental history thus generating hundreds of differentiated cell types in the human body.

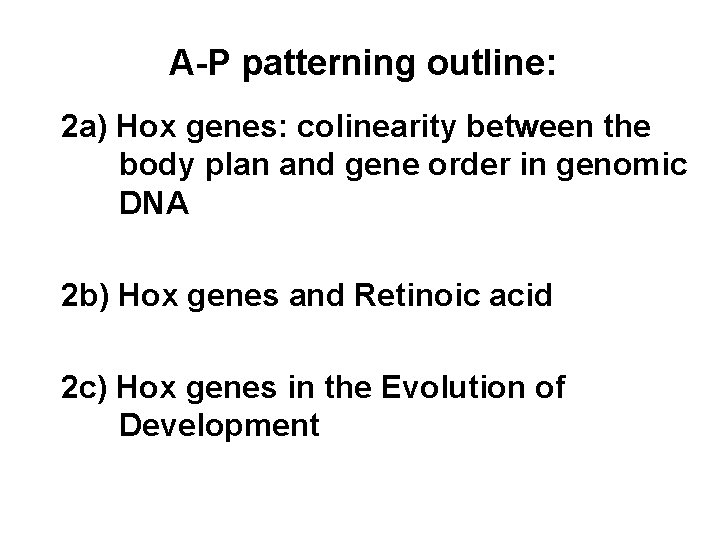
A-P patterning outline: 2 a) Hox genes: colinearity between the body plan and gene order in genomic DNA 2 b) Hox genes and Retinoic acid 2 c) Hox genes in the Evolution of Development

Homeotic Mutations

Cloning of the homeobox (nucleic acid). Homeodomain refers to protein. The homeodomain is a 60 aa DNADefine Hox, homeobox binding domain that is very conserved during evolution. It fits into the major groove of the DNA. The term homeobox is reserved for the nucleic acid sequences that encode homeodomains. Since they are highly conserved, they can be detected by low-stringency hybridization across species.

Homeotic genes specify body segment identity in Drosophila.

Hox complexes are conserved between Drosophila and mammals (from De Robertis et al. , Scientific American, 1990)


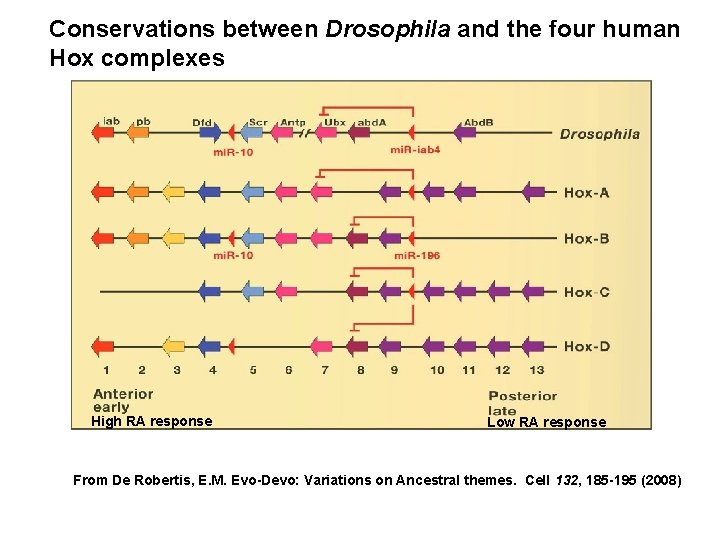
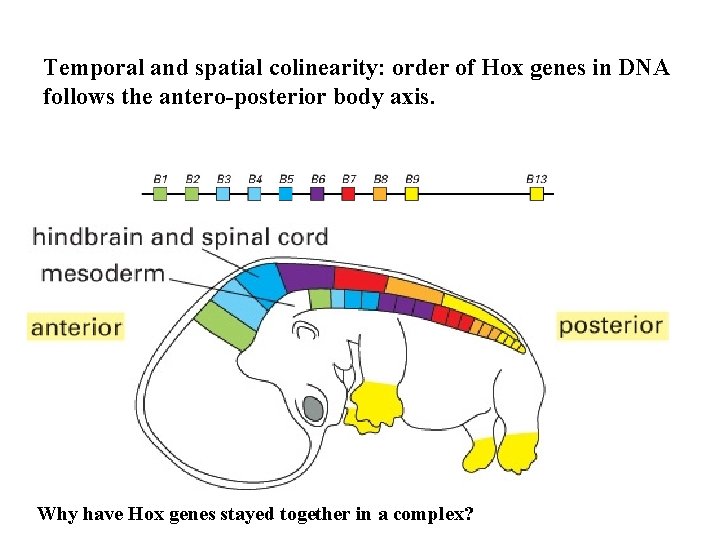
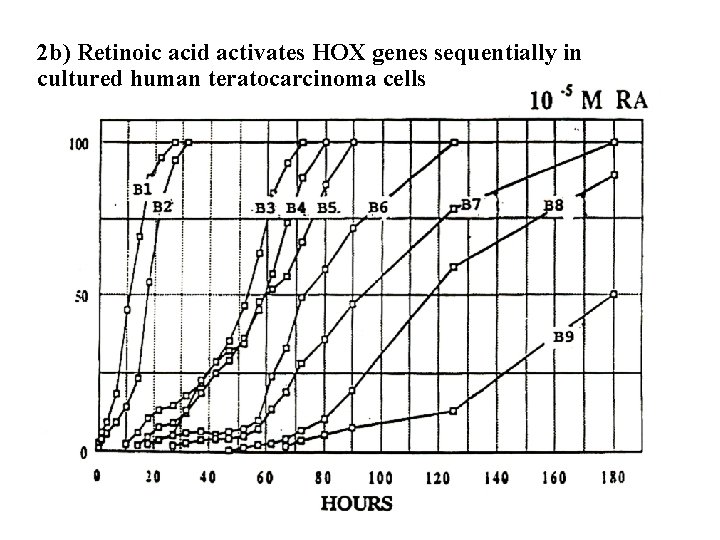
Vertebrates have four Hox complexes, with about 10 genes each. They can be aligned in 13 groups of paralogues. They display colinearity: a) Temporal colinearity: genes on one end of the complex are expressed first, those on the other (posterior) end are turned on last. b) Spatial colinearity: the more anteriorly expressed genes are in one end, the more posterior ones at the other end of the gene complex. c) Anterior Hox genes are activated sequentially by retinoic acid.

Homeobox (Hox) genes were originally discovered in the fruit fly Drosophila, where they function through a conserved omeodomain as transcriptional regulators to control embryonic morphogenesis. Since then over 1000 homeodomain proteins have been identified in several species. In vertebrates, 39 Hox genes have been identified as homologs of the original Drosophila complex, and like their Drosophila counterparts they are organized within chromosomal clusters. Vertebrate Hox genes have also been shown to play a critical role in embryonic development as transcriptional regulators. In vertebrates, more than 200 homeobox-containing genes have been identified

In Drosophila, a single homeotic complex (HOM-C) comprised of two separate clusters [Bithorax complex (BX-C) and Antennapedia complex (ANT-C)] is located on chromosome 3 In mice the Hox complex is comprised of 39 genes that are arranged into four separate chromosomal clusters designated Hox A, B, C, and D Vertebrate Hox and Drosophila HOM-C homeobox clusters is spatial colinearity, which is the expression of the Hox genes along the anteroposterior axis of the embryo in congruence with their arrangement along the chromosome. Thus, the 3’ genes within a chromosomal cluster are expressed more anteriorly than the 5’ genes with respect to the rostrocaudal axis of the embryo. In general, vertebrate Hox genes also display temporal colinearity and colinear sensitivity to RA, a known inducer of Hox gene expression

Ems empty spiracles (Emx in mammalian), transcription factors belonging to the orthodenticle family (Otx 1, Otx 2) play an important role during early and later events required for proper brain development. Otx 1 is involved in corticogenesis, sense organ development and pituitary functions, while Otx 2 is necessary earlier in development, for the correct anterior neural plate specification and organisation of the primitive streak. The first few processes that vertebrate embryos undergo - the division of the egg cytoplasm into cells (cleavage), the formation of the primary tissue layers (gastrulation) and the designation of embryonic coordinates (axis formation) do not seem to require the involvement of Hox genes and other anterior-posterior patterning genes. However, after the initiation of gastrulation, the subdivision of the embryo along the anterior-posterior axis begins. Concurrently, genes like the Hox genes, orthodenticle-related genes, and other homeobox-containing genes, start to be expressed in specific anterior-posterior domains. The Hox genes are expressed from the hindbrain posteriorly and turn on in an ordered sequential fashion, posterior to the domainof Otx 2, an orthodenticle homolog.


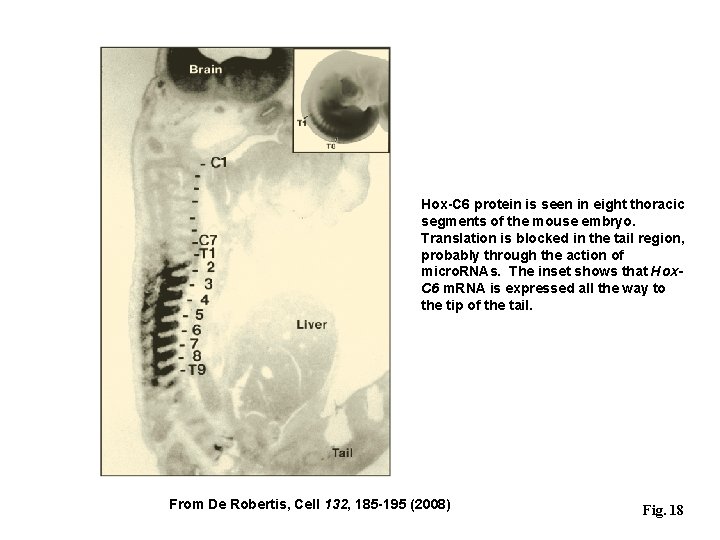
Hox-C 6 protein is seen in eight thoracic segments of the mouse embryo. Translation is blocked in the tail region, probably through the action of micro. RNAs. The inset shows that Hox. C 6 m. RNA is expressed all the way to the tip of the tail. From De Robertis, Cell 132, 185 -195 (2008) Fig. 18

Conservations between Drosophila and the four human Hox complexes High RA response Low RA response From De Robertis, E. M. Evo-Devo: Variations on Ancestral themes. Cell 132, 185 -195 (2008)

Temporal and spatial colinearity: order of Hox genes in DNA follows the antero-posterior body axis. Why have Hox genes stayed together in a complex?

2 b) Retinoic acid activates HOX genes sequentially in cultured human teratocarcinoma cells

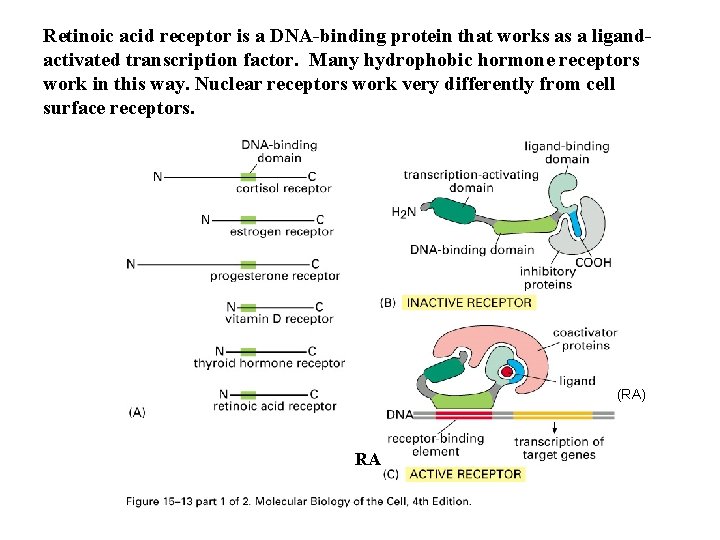
Retinoic acid receptor is a DNA-binding protein that works as a ligandactivated transcription factor. Many hydrophobic hormone receptors work in this way. Nuclear receptors work very differently from cell surface receptors. (RA) RA

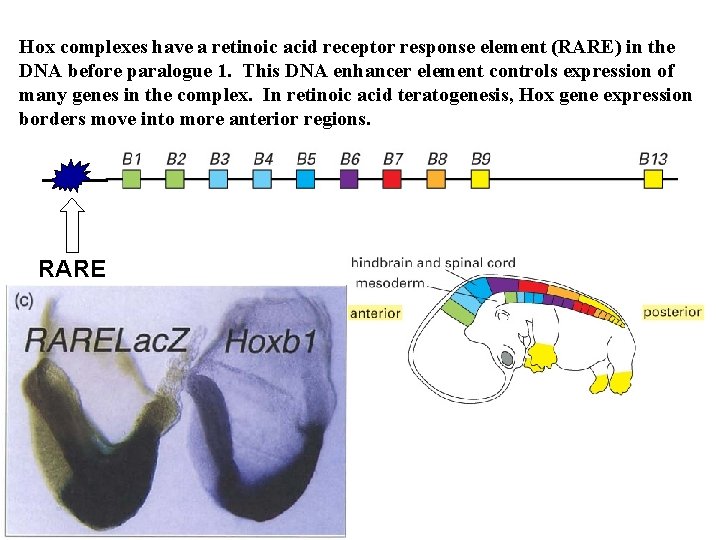
Hox complexes have a retinoic acid receptor response element (RARE) in the DNA before paralogue 1. This DNA enhancer element controls expression of many genes in the complex. In retinoic acid teratogenesis, Hox gene expression borders move into more anterior regions. RARE

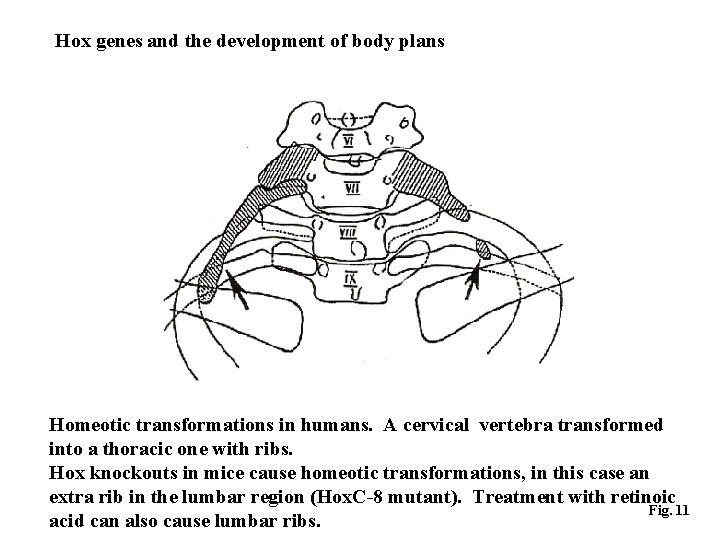
Hox genes and the development of body plans Homeotic transformations in humans. A cervical vertebra transformed into a thoracic one with ribs. Hox knockouts in mice cause homeotic transformations, in this case an extra rib in the lumbar region (Hox. C-8 mutant). Treatment with retinoic Fig. 11 acid can also cause lumbar ribs.








Numb vs Notch: drosophila vs Uomo


 Hox gene mutation in drosophila
Hox gene mutation in drosophila Hox gene mutation in drosophila
Hox gene mutation in drosophila What are homeotic genes
What are homeotic genes Genes hox
Genes hox Geni hox
Geni hox Genes hox
Genes hox Genes hox
Genes hox Hox gene
Hox gene Odd hox
Odd hox Hox
Hox Geni hox
Geni hox Physical state of covalent compounds
Physical state of covalent compounds Ionic covalent metallic
Ionic covalent metallic Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Introductory phrases for quotes
Introductory phrases for quotes Discourse analysis and vocabulary
Discourse analysis and vocabulary Discourse analysis and vocabulary
Discourse analysis and vocabulary What action is the ground guide signaling?
What action is the ground guide signaling? 3 types of cell signaling
3 types of cell signaling Respond to
Respond to Chapter 11 cell communication
Chapter 11 cell communication Three stages of cell signaling
Three stages of cell signaling Ligand signaling molecule
Ligand signaling molecule Signaling system 7
Signaling system 7 Autocrine and juxtacrine signaling
Autocrine and juxtacrine signaling Autocrine and juxtacrine signaling
Autocrine and juxtacrine signaling Chemical signaling
Chemical signaling Exocrine cell signaling
Exocrine cell signaling Phosphorylation cascade
Phosphorylation cascade Chemical signaling
Chemical signaling Chemical signaling
Chemical signaling Most embryonic industries emerge from:
Most embryonic industries emerge from: Friendship warmth touch
Friendship warmth touch Chapter 48 neurons synapses and signaling
Chapter 48 neurons synapses and signaling Cell signaling
Cell signaling Cell signaling overview
Cell signaling overview Pdi cell signaling
Pdi cell signaling Scp 3710
Scp 3710 Use visual signaling techniques
Use visual signaling techniques Chapter 48 neurons synapses and signaling
Chapter 48 neurons synapses and signaling Frontiers in bioscience
Frontiers in bioscience Plant growth analysis
Plant growth analysis