Molecular Partition Function July 1998 Guna Hua CHEN

- Slides: 18

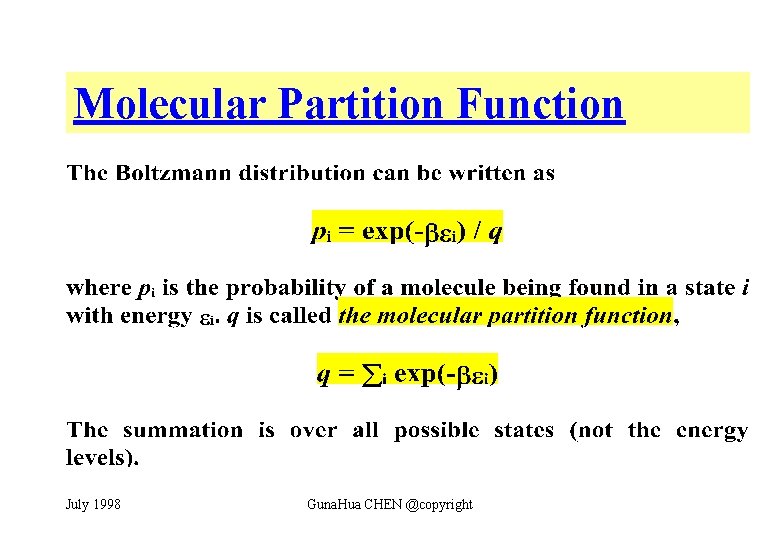
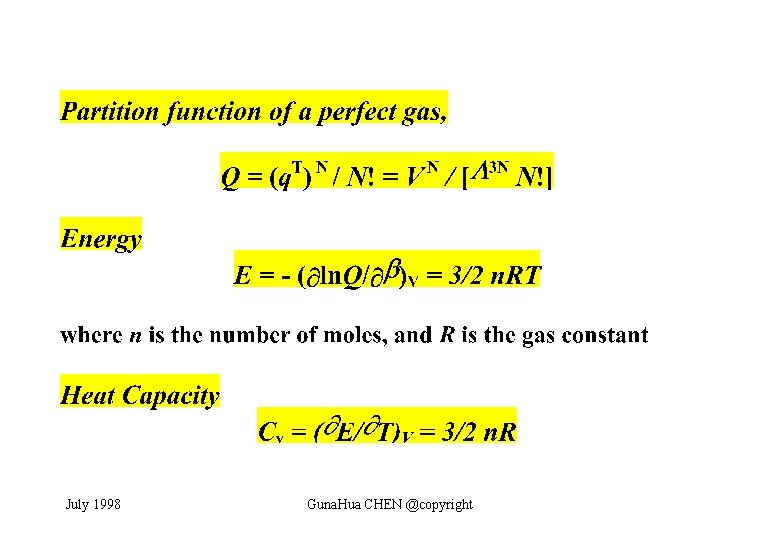
Molecular Partition Function July 1998 Guna. Hua CHEN @copyright

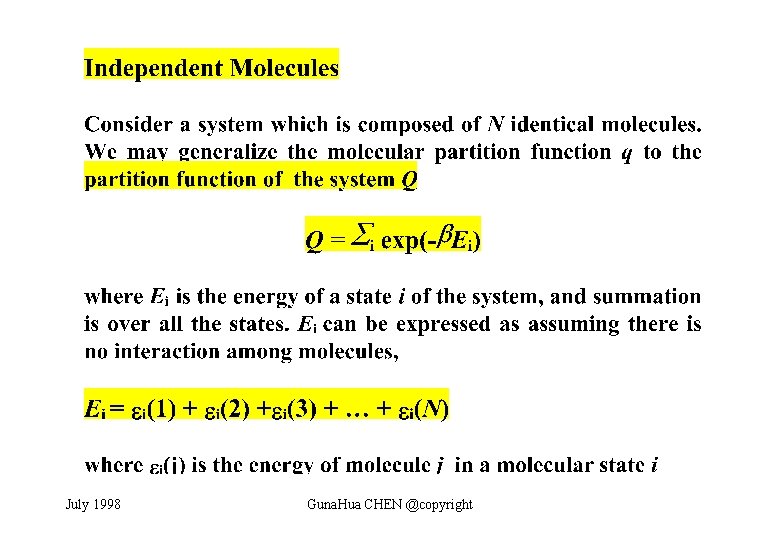
July 1998 Guna. Hua CHEN @copyright

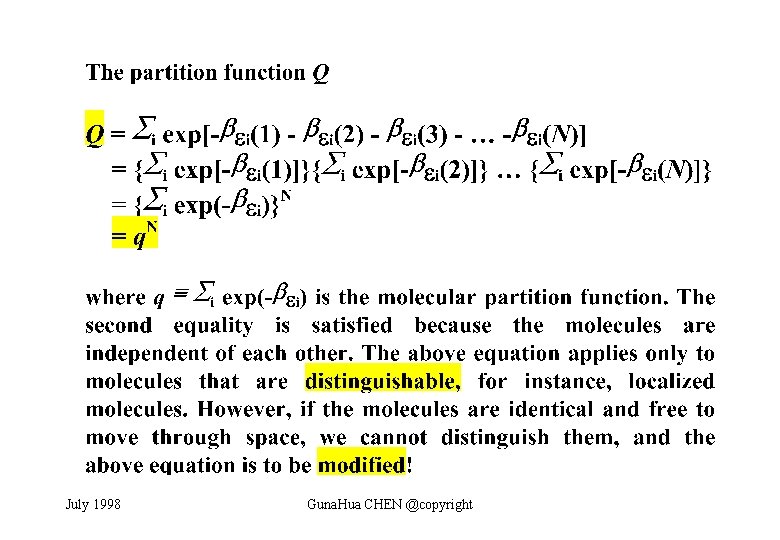
July 1998 Guna. Hua CHEN @copyright

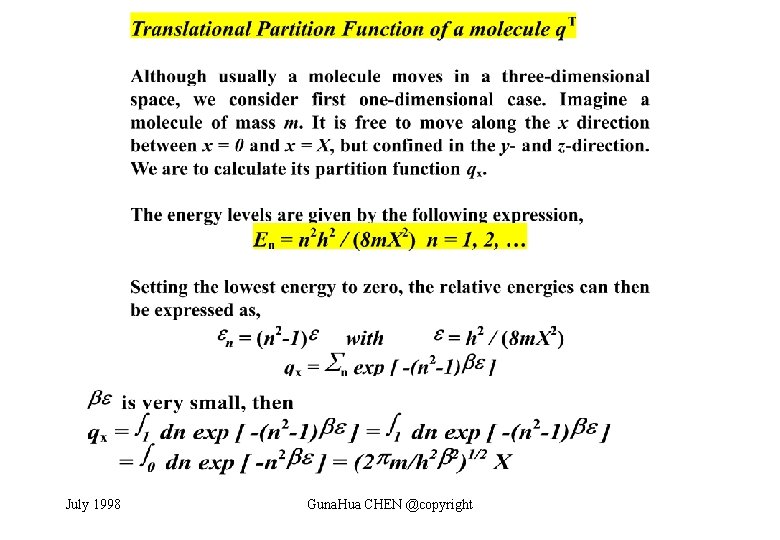
July 1998 Guna. Hua CHEN @copyright

July 1998 Guna. Hua CHEN @copyright

July 1998 Guna. Hua CHEN @copyright

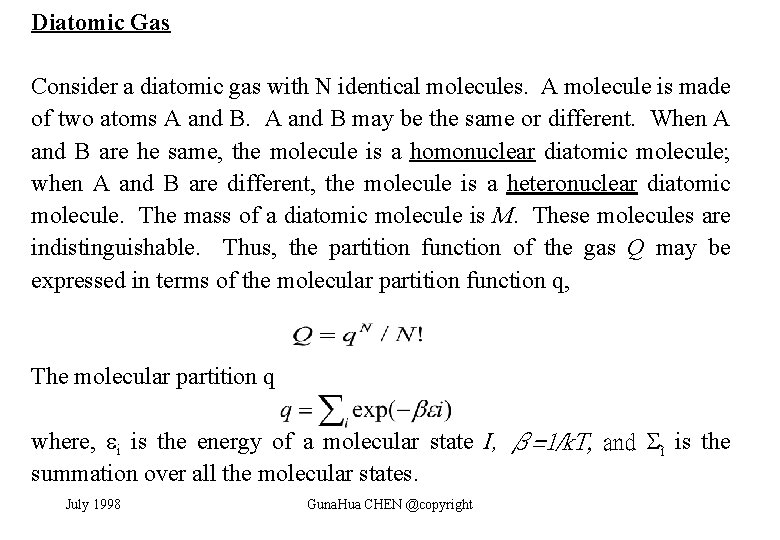
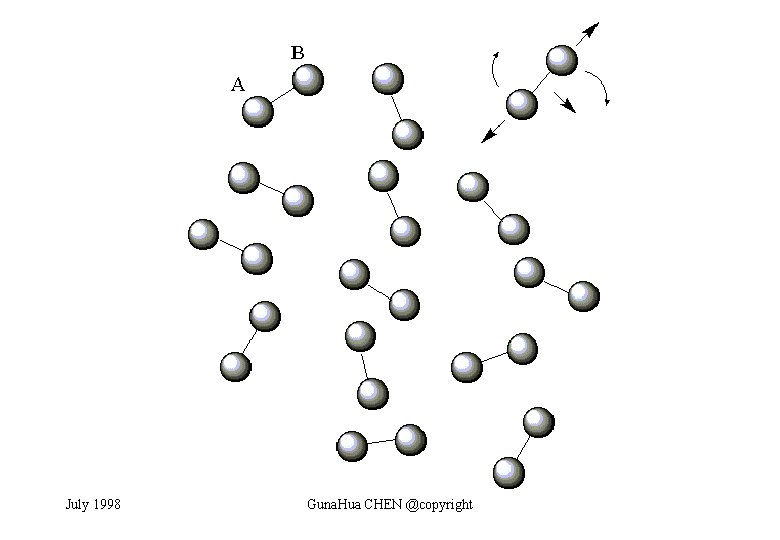
Diatomic Gas Consider a diatomic gas with N identical molecules. A molecule is made of two atoms A and B may be the same or different. When A and B are he same, the molecule is a homonuclear diatomic molecule; when A and B are different, the molecule is a heteronuclear diatomic molecule. The mass of a diatomic molecule is M. These molecules are indistinguishable. Thus, the partition function of the gas Q may be expressed in terms of the molecular partition function q, The molecular partition q where, i is the energy of a molecular state I, β=1/k. T, and ì is the summation over all the molecular states. July 1998 Guna. Hua CHEN @copyright

July 1998 Guna. Hua CHEN @copyright

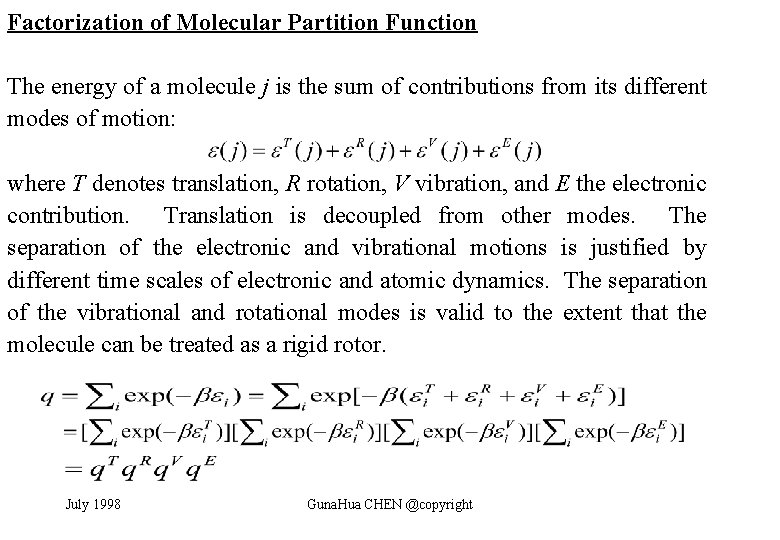
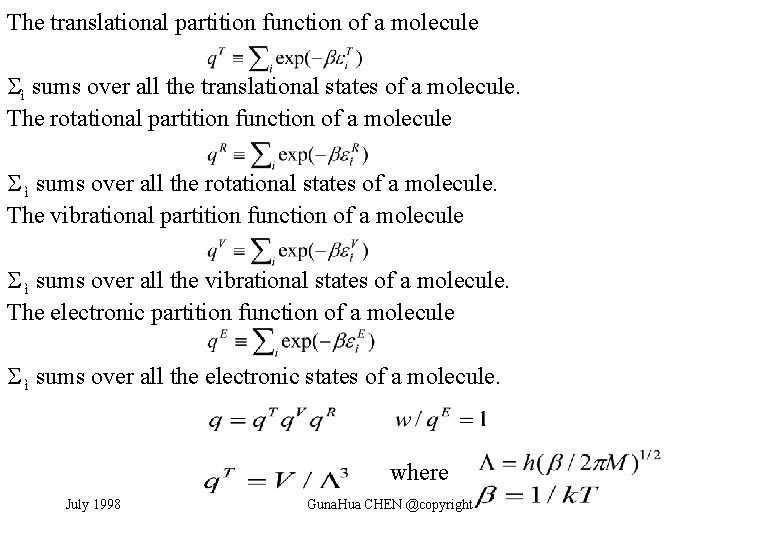
Factorization of Molecular Partition Function The energy of a molecule j is the sum of contributions from its different modes of motion: where T denotes translation, R rotation, V vibration, and E the electronic contribution. Translation is decoupled from other modes. The separation of the electronic and vibrational motions is justified by different time scales of electronic and atomic dynamics. The separation of the vibrational and rotational modes is valid to the extent that the molecule can be treated as a rigid rotor. July 1998 Guna. Hua CHEN @copyright

The translational partition function of a molecule ì sums over all the translational states of a molecule. The rotational partition function of a molecule ì sums over all the rotational states of a molecule. The vibrational partition function of a molecule ì sums over all the vibrational states of a molecule. The electronic partition function of a molecule ì sums over all the electronic states of a molecule. where July 1998 Guna. Hua CHEN @copyright

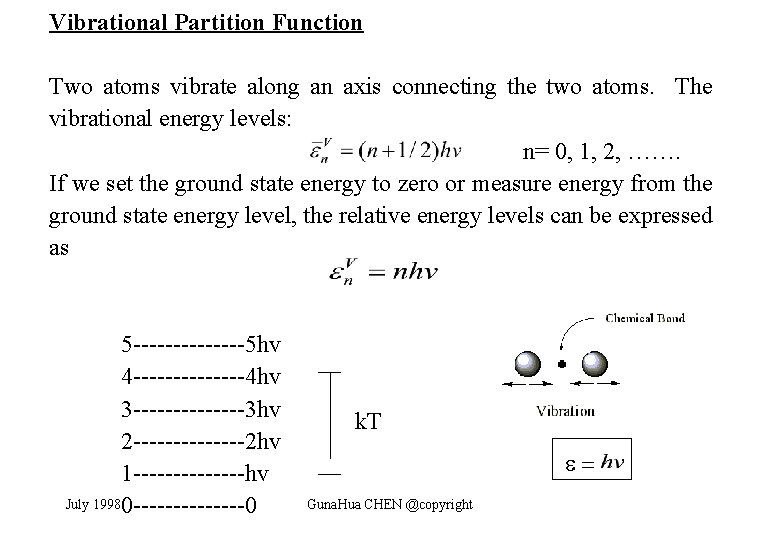
Vibrational Partition Function Two atoms vibrate along an axis connecting the two atoms. The vibrational energy levels: n= 0, 1, 2, ……. If we set the ground state energy to zero or measure energy from the ground state energy level, the relative energy levels can be expressed as 5 -------5 hv 4 -------4 hv 3 -------3 hv 2 -------2 hv 1 -------hv July 1998 0 -------0 k. T = Guna. Hua CHEN @copyright

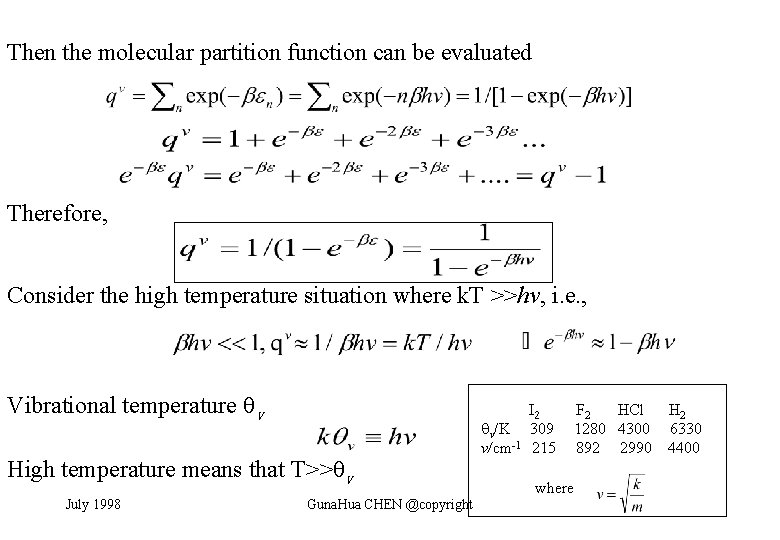
Then the molecular partition function can be evaluated Therefore, Consider the high temperature situation where k. T >>hv, i. e. , Vibrational temperature v High temperature means that T>> v July 1998 Guna. Hua CHEN @copyright v/K v/cm-1 I 2 309 215 F 2 HCl 1280 4300 892 2990 where H 2 6330 4400

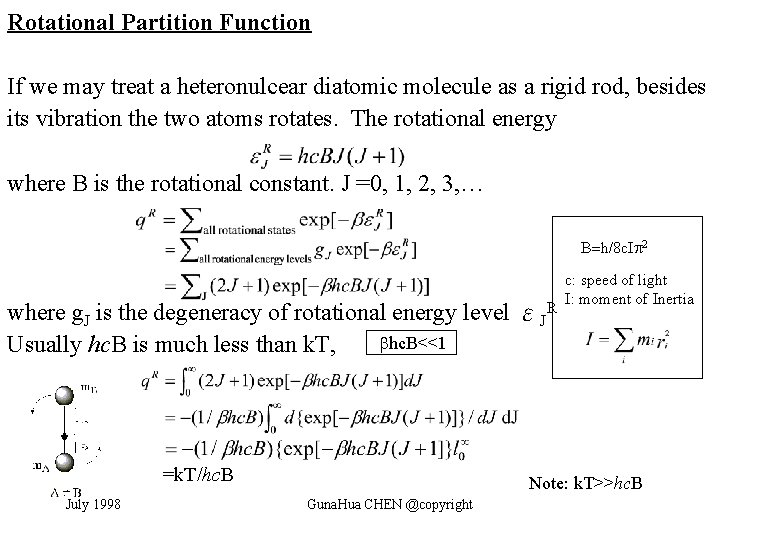
Rotational Partition Function If we may treat a heteronulcear diatomic molecule as a rigid rod, besides its vibration the two atoms rotates. The rotational energy where B is the rotational constant. J =0, 1, 2, 3, … B=h/8 c. I 2 where g. J is the degeneracy of rotational energy level εJR hc. B<<1 Usually hc. B is much less than k. T, =k. T/hc. B July 1998 c: speed of light I: moment of Inertia Note: k. T>>hc. B Guna. Hua CHEN @copyright

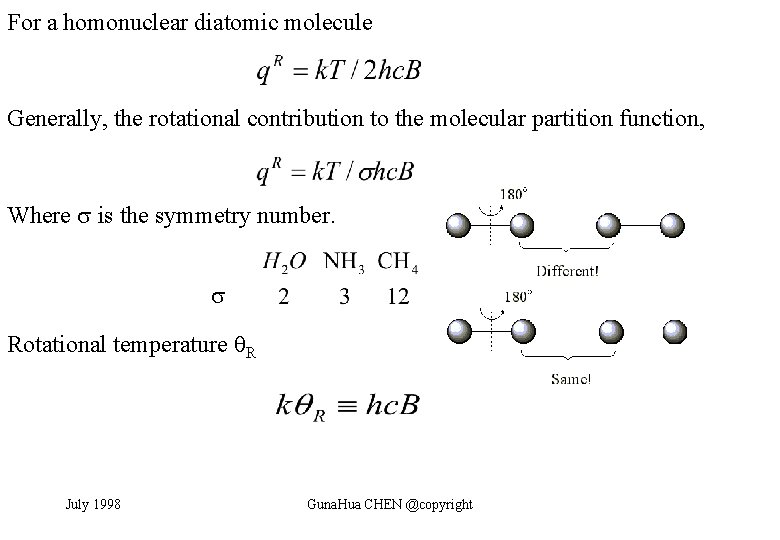
For a homonuclear diatomic molecule Generally, the rotational contribution to the molecular partition function, Where is the symmetry number. Rotational temperature R July 1998 Guna. Hua CHEN @copyright

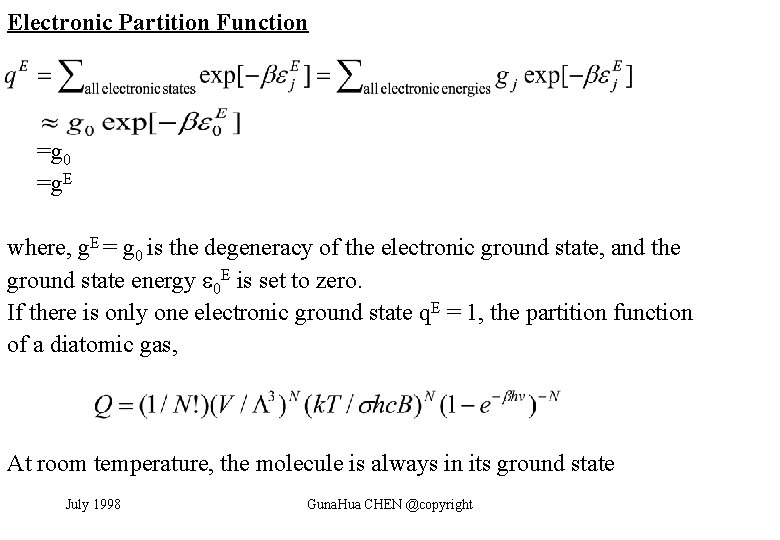
Electronic Partition Function =g 0 =g. E where, g. E = g 0 is the degeneracy of the electronic ground state, and the ground state energy 0 E is set to zero. If there is only one electronic ground state q. E = 1, the partition function of a diatomic gas, At room temperature, the molecule is always in its ground state July 1998 Guna. Hua CHEN @copyright

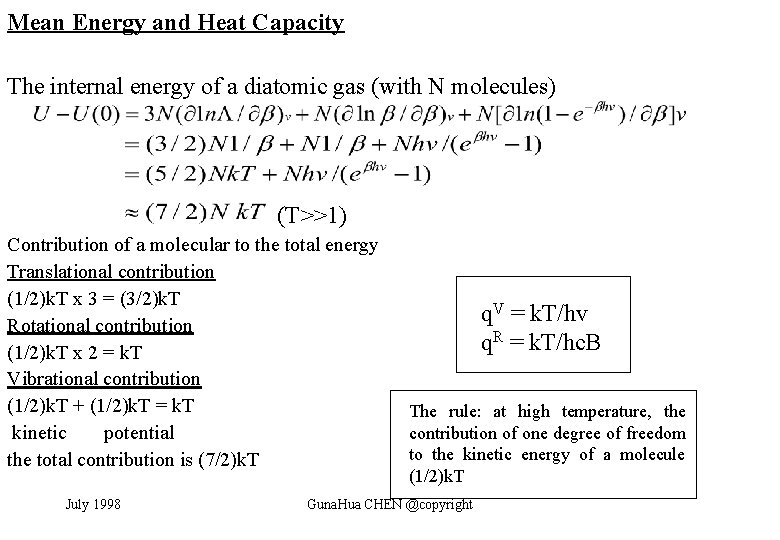
Mean Energy and Heat Capacity The internal energy of a diatomic gas (with N molecules) (T>>1) Contribution of a molecular to the total energy Translational contribution (1/2)k. T x 3 = (3/2)k. T Rotational contribution (1/2)k. T x 2 = k. T Vibrational contribution (1/2)k. T + (1/2)k. T = k. T kinetic potential the total contribution is (7/2)k. T July 1998 q. V = k. T/hv q. R = k. T/hc. B The rule: at high temperature, the contribution of one degree of freedom to the kinetic energy of a molecule (1/2)k. T Guna. Hua CHEN @copyright

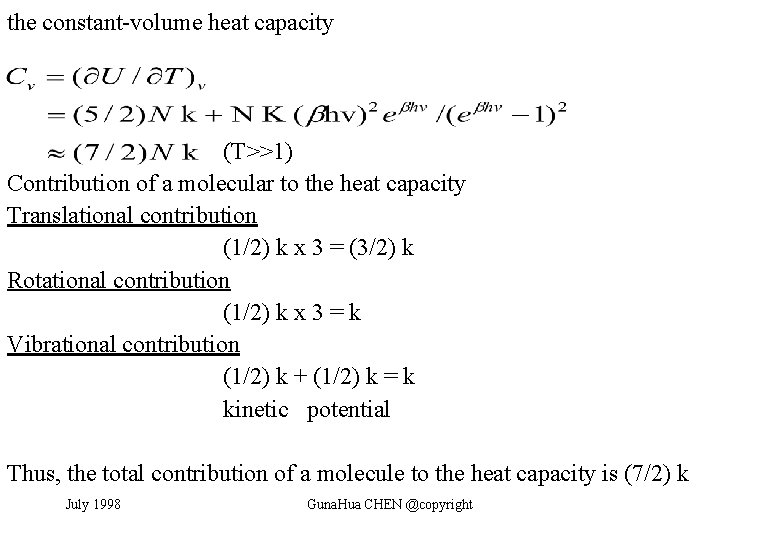
the constant-volume heat capacity (T>>1) Contribution of a molecular to the heat capacity Translational contribution (1/2) k x 3 = (3/2) k Rotational contribution (1/2) k x 3 = k Vibrational contribution (1/2) k + (1/2) k = k kinetic potential Thus, the total contribution of a molecule to the heat capacity is (7/2) k July 1998 Guna. Hua CHEN @copyright

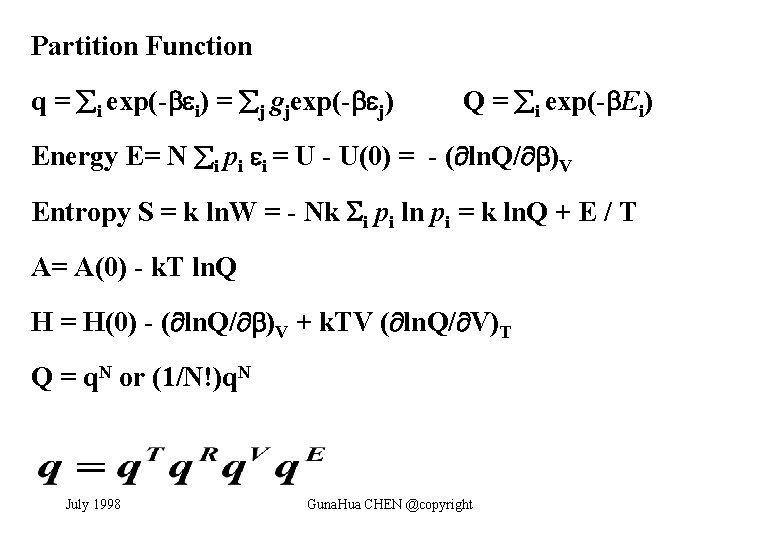
Partition Function q = i exp(- i) = j gjexp(- j) Q = i exp(- Ei) Energy E= N i pi i = U - U(0) = - ( ln. Q/ )V Entropy S = k ln. W = - Nk i pi ln pi = k ln. Q + E / T A= A(0) - k. T ln. Q H = H(0) - ( ln. Q/ )V + k. TV ( ln. Q/ V)T Q = q. N or (1/N!)q. N July 1998 Guna. Hua CHEN @copyright