Molecular Neurobiology of the Nematode Caenorhabditis elegans Marvin
Molecular Neurobiology of the Nematode Caenorhabditis elegans Marvin L. Bayne, Ph. D. HS 333, mbayne@drew. edu
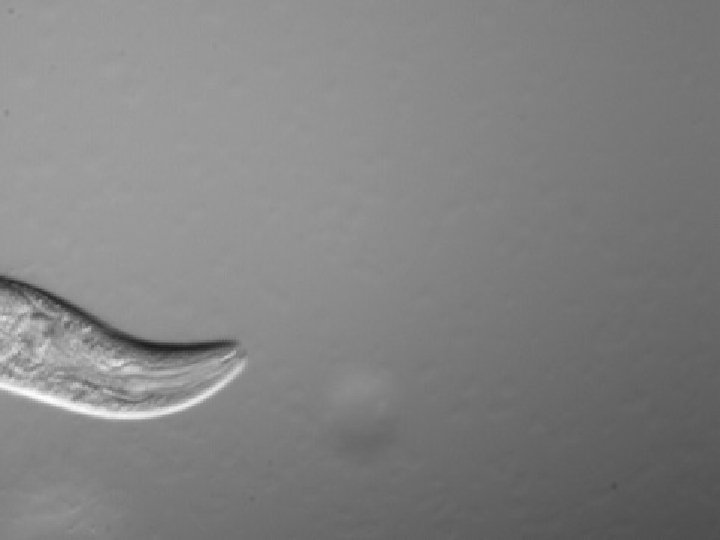

Why Study Worms? Sydney Brenner “Thus we want a multicellular organism which has a short life cycle, can be easily cultivated, and is small enough to be handled in large numbers, like a micro-organism. It should have relatively few cells, so that exhaustive studies of lineage and patterns can be made, and should be amenable to genetic analysis. ” --Excerpts from Proposal to the Medical Research Council, 1963
C. elegans as a Model System • Easy to cultivate – Small: ~1 mm in length – Grown on agar plates of E. coli, scaled up in liquid culture – Large brood size: ~300; short generation time: ~3 days • Genetic analysis – Forward Genetics: • EMS mutagenesis, transposons mutagenesis • Self fertilizing hermaphrodites allows easy clonal expansion – Reverse Genetics: • Gene Knockouts/Replacements: Transgenesis; CRISPR/Cas-9 • Gene Knockdowns thru RNAi bacterial delivery system • Transparency – Allowed cell lineage mapping of all 959 cells – Allows use of Green Fluorescent Protein tagged promoter fusions and proteins to follow expression in vivo • Can be used to Model Human Diseases – ~ 60 -80% of C. elegans genes have human counterparts – ~42% of human disease genes have C. elegans counterparts – Can generate “humanized” worms; replace worm gene with human counterpart
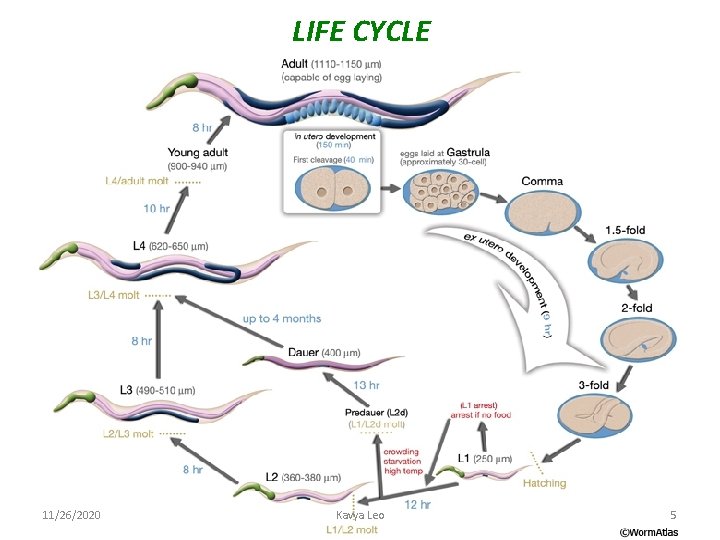
LIFE CYCLE 11/26/2020 Kavya Leo 5
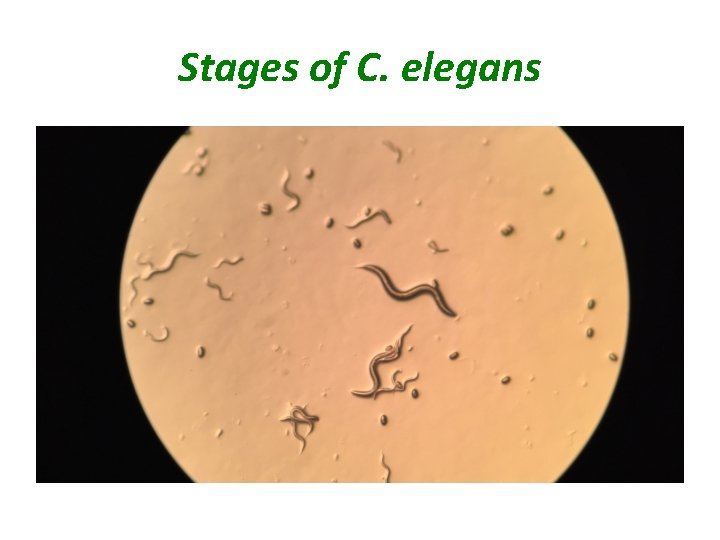
Stages of C. elegans

C. Elegans Milestones • 1963: Brenner proposal to the MRC • 1998: First multicellular organism fully sequenced • 2002: Cell Lineage Nobel Prize to Brenner, Horvitz and Saulston • 2006: RNAi Discovery Nobel Prize to Fire and Mello • 2008: Nobel Prize to Chalfie for GFP in C. elegans • 2011: First connectosome completed

Nervous System of C. elegans • 302 neurons, 32 chemosensory neurons, 8 dopamine neurons – Function of individual neurons determined by laser ablation • Complete “connectosome”determined – 6393 chemical synapses – 1410 neuromuscular junctions – 890 gap junctions

Bayne Lab 2017 -2018 Aidan Antonelli, Alina Qasim, Stephanie De. Fonzo, Lexi Holroyd, Katie Revelas, Leanne Fogarty • Currently 6 students – 4 sophomores, 1 junior, 1 senior • Current Projects – Parkinson’s Disease – Cystic Fibrosis – Autism Spectrum Disorder
Parkinson’s Disease Parkinson’s disease is a degenerative neurological disease affecting dopamine producing neurons Damage to dopaminergic neurons can be caused by genetic defects, environmental factors such as exposure to neurotoxins like pesticides, or traumatic brain injury. Symptoms include tremors, slowness of movements, gait problems
C. elegans Models of Parkinson's Disease • Genetic: C. elegans strains expressing human PD related genes resulting in age-dependent degeneration of dopaminergic neurons – Alpha-synuclein: normal and A 53 T mutation – LRRK 2: G 2019 S mutation • Neurotoxins: chemical degeneration of dopamine neurons – MPTP (1 -methyl-4 -phenyl-1, 2, 3, 6 -tetrahydropyridine) – 6 -OHDA (6 -hydroxyl dopamine) • Degeneration of dopamine neurons can be monitored using worms expressing Green Fluorescent Protein specifically in the 8 dopaminergic neurons • Worms develop movement abnormalities modeling movement disorders in Parkinson’s disease patients – Swim to crawl paralysis • We are using these models to identify drugs and/or genes that protect dopamine neurons
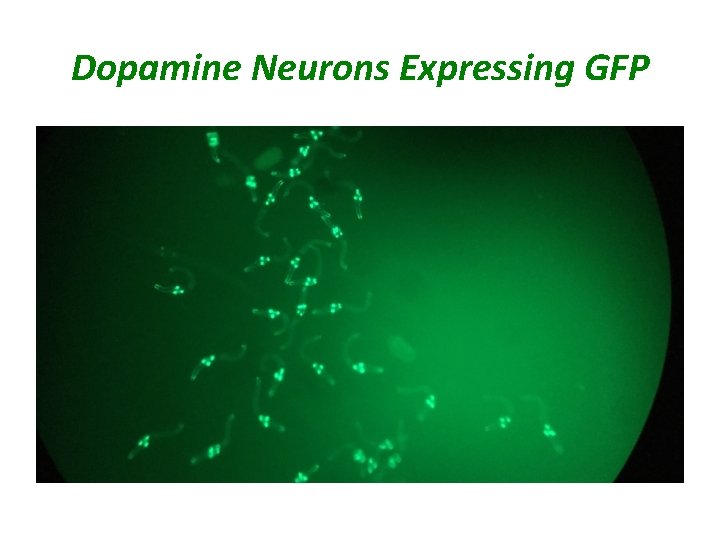
Dopamine Neurons Expressing GFP
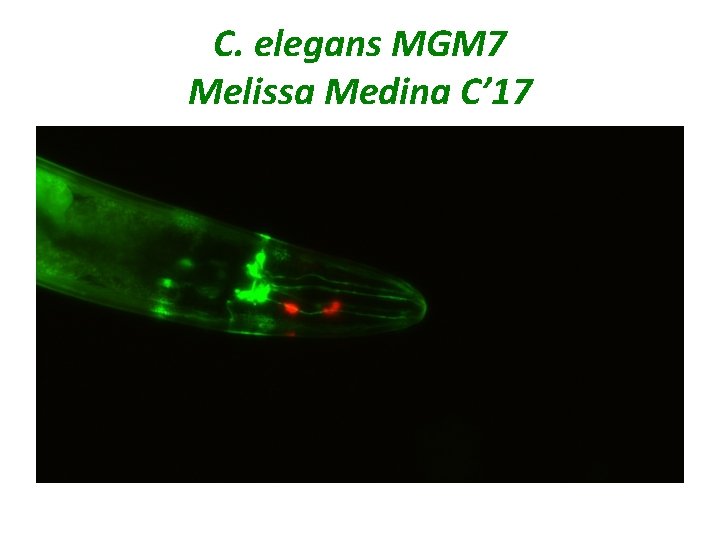
C. elegans MGM 7 Melissa Medina C’ 17
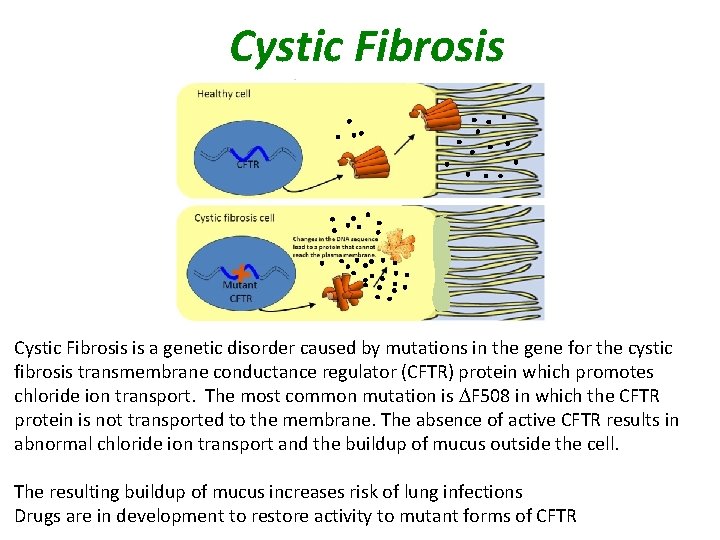
Cystic Fibrosis is a genetic disorder caused by mutations in the gene for the cystic fibrosis transmembrane conductance regulator (CFTR) protein which promotes chloride ion transport. The most common mutation is DF 508 in which the CFTR protein is not transported to the membrane. The absence of active CFTR results in abnormal chloride ion transport and the buildup of mucus outside the cell. The resulting buildup of mucus increases risk of lung infections Drugs are in development to restore activity to mutant forms of CFTR
C. elegans Model of Cystic Fibrosis • Insertion of 6 amino acids from human CFTR into a C. elegans ABC transporter results in normal membrane localization. • Insertion of 5 amino acids representing the DF 508 mutation associated with human CF results in impaired membrane localization. • Membrane localization is monitored in living worms using Red Fluorescent Protein tag. • We are testing compounds from the Cystic Fibrosis Foundation for the ability to restore normal membrane localization to the DF 508 strain.
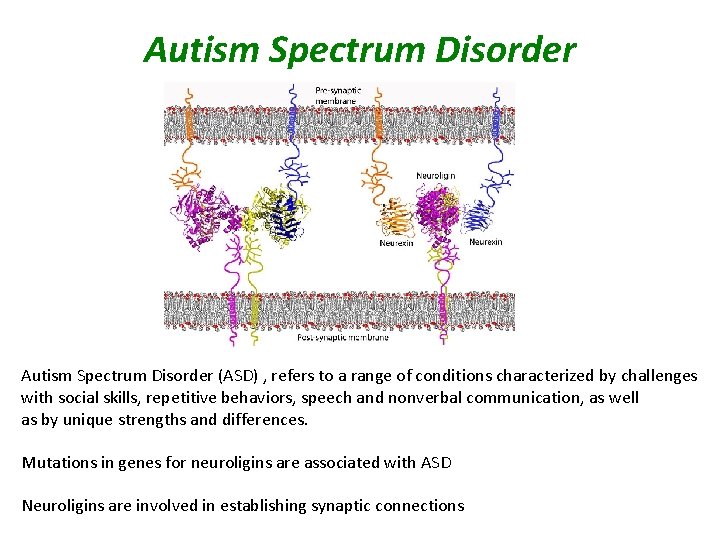
Autism Spectrum Disorder (ASD) , refers to a range of conditions characterized by challenges with social skills, repetitive behaviors, speech and nonverbal communication, as well as by unique strengths and differences. Mutations in genes for neuroligins are associated with ASD Neuroligins are involved in establishing synaptic connections
C. Elegans Model for Autism Spectrum Disorder • Mutations in human neuroligin genes are associated with Autism Spectrum Disorder • Deletion of the C. elegans neuroligin gene results in sensory deficits – Increased sensitivity to mercury – Lack of chemotaxis response to 1 -octanol • C. elegans mutants can be rescued by human wild type neuroligin genes but not by genes carrying mutations associated with ASD • We plan to clone the human neuroligin 3 gene, introduce the R 451 C and G 221 R mutations and generate humanized C. elegans strains using CRISPR/Cas 9; then look for genes or compounds to reverse the effects of the mutations.
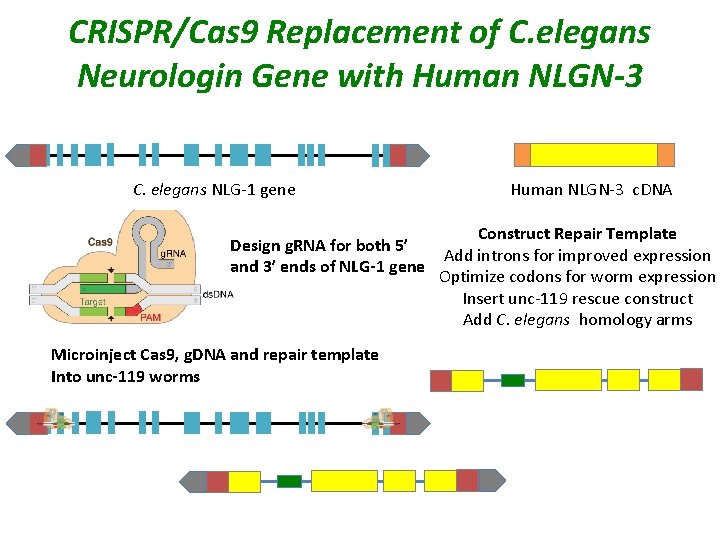
CRISPR/Cas 9 Replacement of C. elegans Neurologin Gene with Human NLGN-3 C. elegans NLG-1 gene Design g. RNA for both 5’ and 3’ ends of NLG-1 gene Microinject Cas 9, g. DNA and repair template Into unc-119 worms Human NLGN-3 c. DNA Construct Repair Template Add introns for improved expression Optimize codons for worm expression Insert unc-119 rescue construct Add C. elegans homology arms

Acknowledgements • Former Students – – – Tanvi Joshi Alice Vinogradsky Krishna Patel Melissa Medina Erin Connors • Advice – Dr. Diane Levitan • C. elegans strains – – C. elegans Genetic Stock Center Dr. Jeremy Van Raamsdonk Dr. Guy Caldwell Dr. Villu Maricq • Support Mumbai, India Princeton Class of 2020 Drew Class of 2017 Drew Class of 2018 Merck Research Labs University of Minnesota Van Andle Institute University of Alabama University of Utah – Charles A. Dana Research Institute for Scientists Emeriti – New England Biolabs Educational Course Support
- Slides: 19