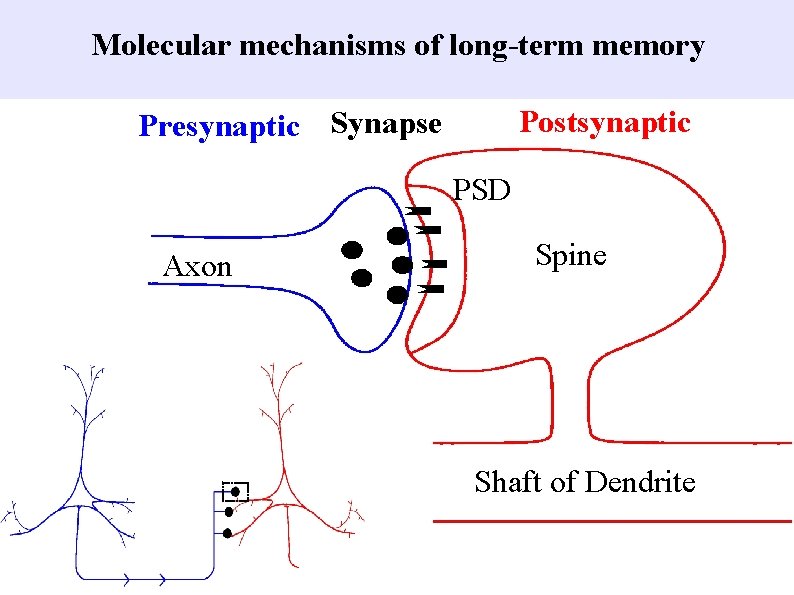
Molecular mechanisms of longterm memory Postsynaptic Presynaptic Synapse
![Bistability in total phosphorylation of Ca. MKII [Ca 2+]=0. 1 M (basal level) Total Bistability in total phosphorylation of Ca. MKII [Ca 2+]=0. 1 M (basal level) Total](https://slidetodoc.com/presentation_image_h/164272142cf4aece0067d33c5f32ace7/image-7.jpg)
![Phosphorylation dominates at high calcium [Ca 2+] = 2 M (for LTP) Total reaction Phosphorylation dominates at high calcium [Ca 2+] = 2 M (for LTP) Total reaction](https://slidetodoc.com/presentation_image_h/164272142cf4aece0067d33c5f32ace7/image-8.jpg)
![How to get bistability 1) Autocatalysis: k+ increases with [C] 2) Saturation: total rate How to get bistability 1) Autocatalysis: k+ increases with [C] 2) Saturation: total rate](https://slidetodoc.com/presentation_image_h/164272142cf4aece0067d33c5f32ace7/image-10.jpg)

- Slides: 66

Molecular mechanisms of long-term memory Postsynaptic Presynaptic Synapse PSD Axon Spine Shaft of Dendrite

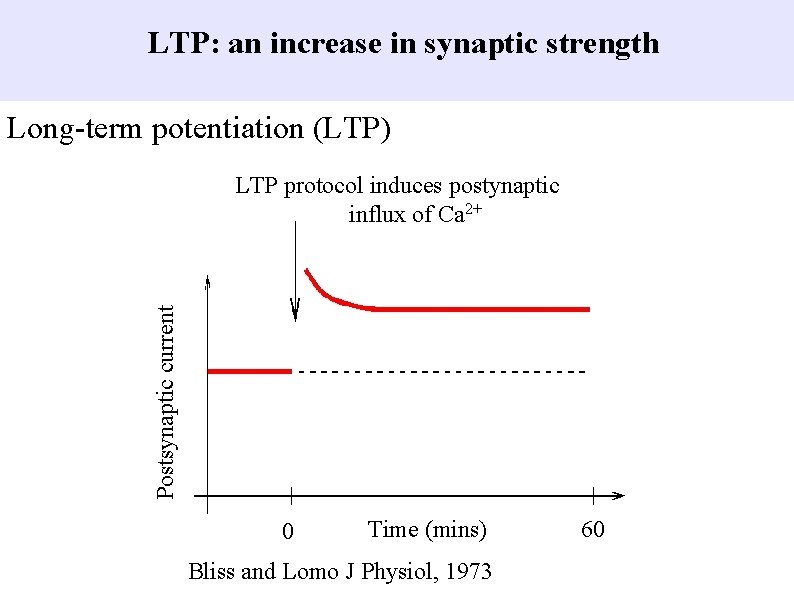
LTP: an increase in synaptic strength Long-term potentiation (LTP) Postsynaptic current LTP protocol induces postynaptic influx of Ca 2+ 0 Time (mins) Bliss and Lomo J Physiol, 1973 60

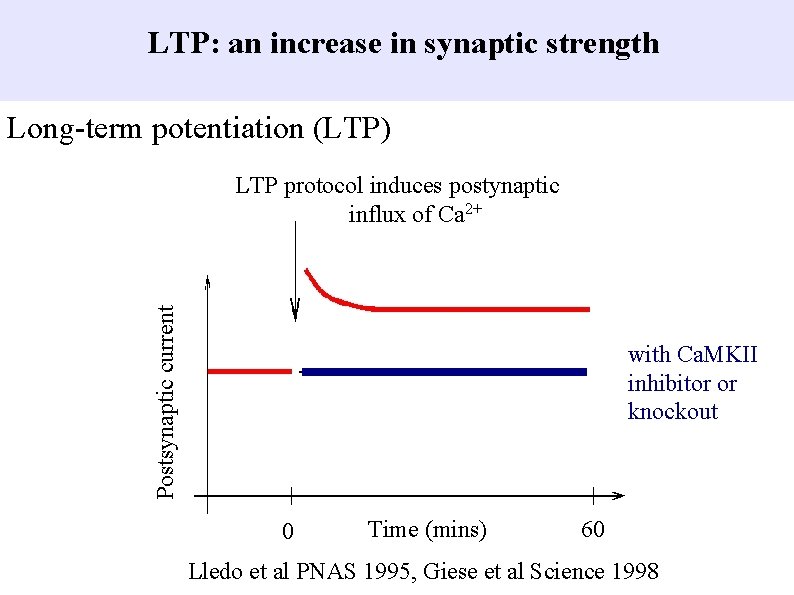
LTP: an increase in synaptic strength Long-term potentiation (LTP) Postsynaptic current LTP protocol induces postynaptic influx of Ca 2+ with Ca. MKII inhibitor or knockout 0 Time (mins) 60 Lledo et al PNAS 1995, Giese et al Science 1998

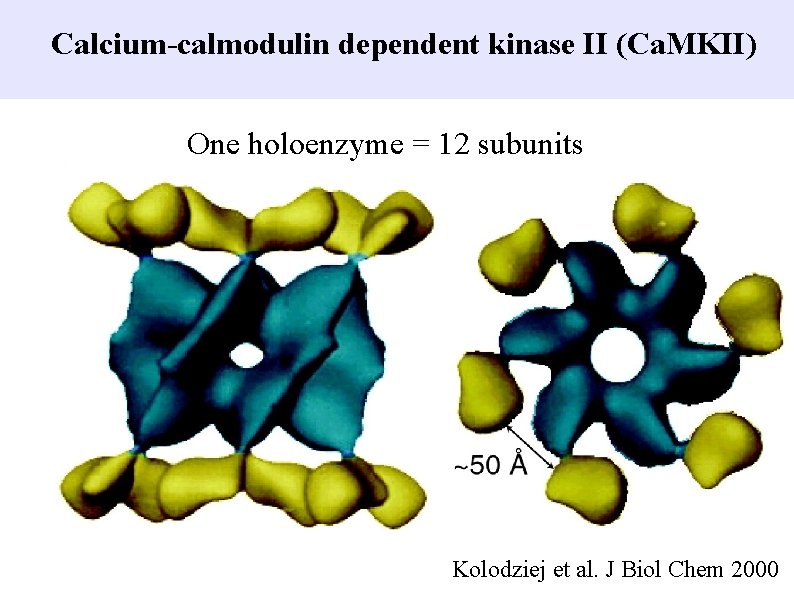
Calcium-calmodulin dependent kinase II (Ca. MKII) One holoenzyme = 12 subunits Kolodziej et al. J Biol Chem 2000

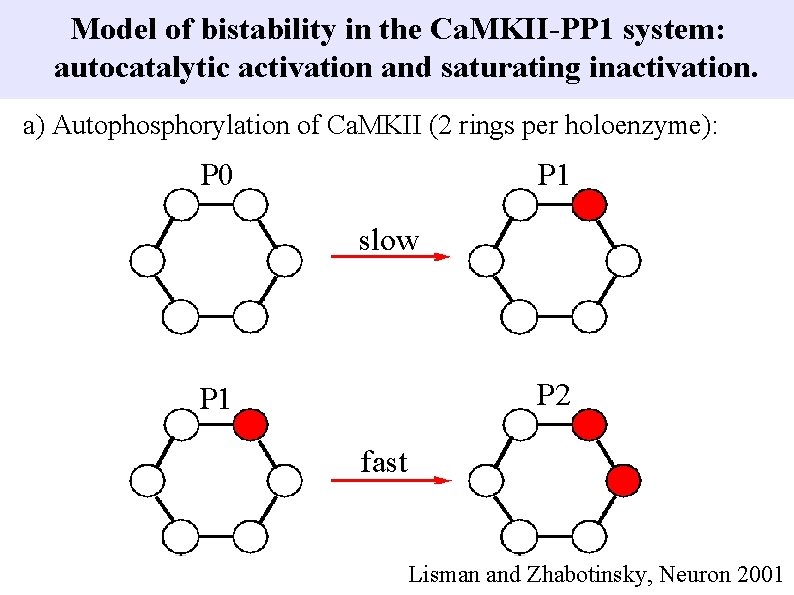
Model of bistability in the Ca. MKII-PP 1 system: autocatalytic activation and saturating inactivation. a) Autophosphorylation of Ca. MKII (2 rings per holoenzyme): P 0 P 1 slow P 2 P 1 fast Lisman and Zhabotinsky, Neuron 2001

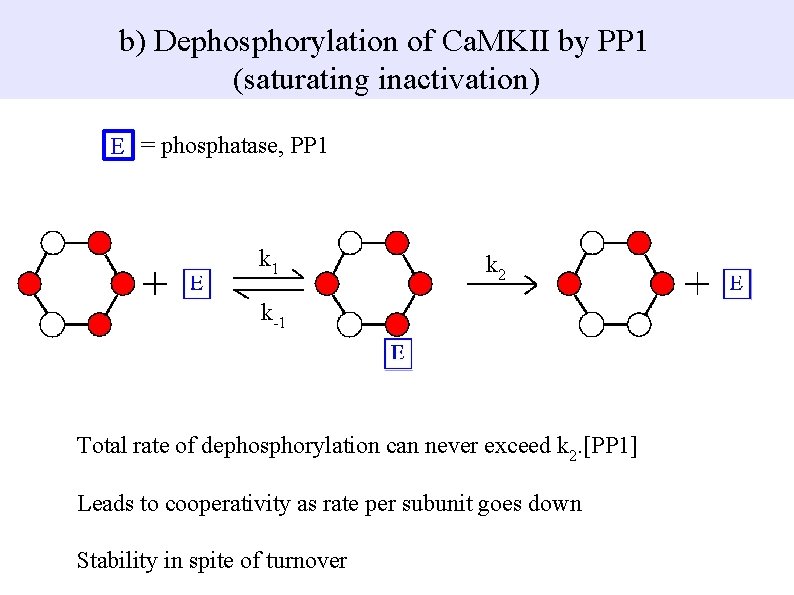
b) Dephosphorylation of Ca. MKII by PP 1 (saturating inactivation) E = phosphatase, PP 1 k 2 k-1 Total rate of dephosphorylation can never exceed k 2. [PP 1] Leads to cooperativity as rate per subunit goes down Stability in spite of turnover
![Bistability in total phosphorylation of Ca MKII Ca 20 1 M basal level Total Bistability in total phosphorylation of Ca. MKII [Ca 2+]=0. 1 M (basal level) Total](https://slidetodoc.com/presentation_image_h/164272142cf4aece0067d33c5f32ace7/image-7.jpg)
Bistability in total phosphorylation of Ca. MKII [Ca 2+]=0. 1 M (basal level) Total reaction rate Rate of dephosphoryation Rate of phosphorylation 0 0 No. of active subunits 12 N
![Phosphorylation dominates at high calcium Ca 2 2 M for LTP Total reaction Phosphorylation dominates at high calcium [Ca 2+] = 2 M (for LTP) Total reaction](https://slidetodoc.com/presentation_image_h/164272142cf4aece0067d33c5f32ace7/image-8.jpg)
Phosphorylation dominates at high calcium [Ca 2+] = 2 M (for LTP) Total reaction rate Rate of dephosphoryation Rate of phosphorylation 0 0 No. of active subunits 12 N

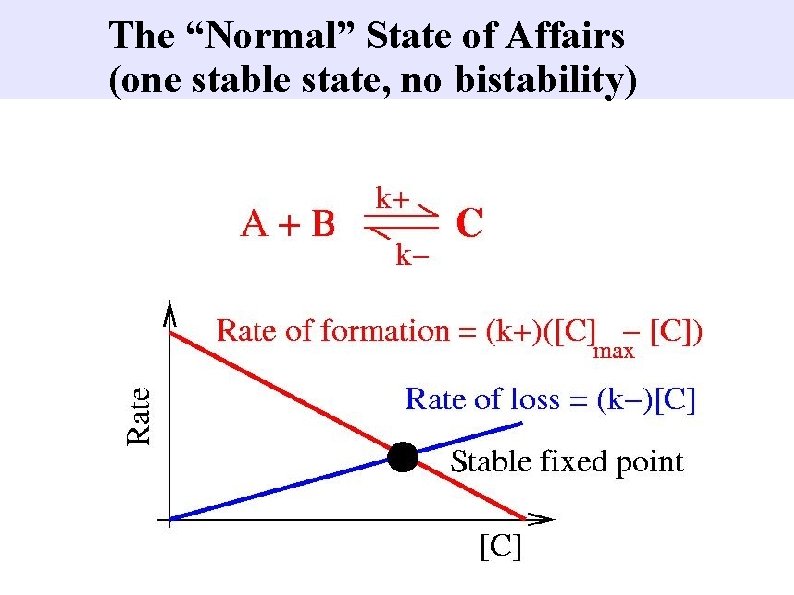
The “Normal” State of Affairs (one stable state, no bistability)
![How to get bistability 1 Autocatalysis k increases with C 2 Saturation total rate How to get bistability 1) Autocatalysis: k+ increases with [C] 2) Saturation: total rate](https://slidetodoc.com/presentation_image_h/164272142cf4aece0067d33c5f32ace7/image-10.jpg)
How to get bistability 1) Autocatalysis: k+ increases with [C] 2) Saturation: total rate down, (k-)[C], is limited

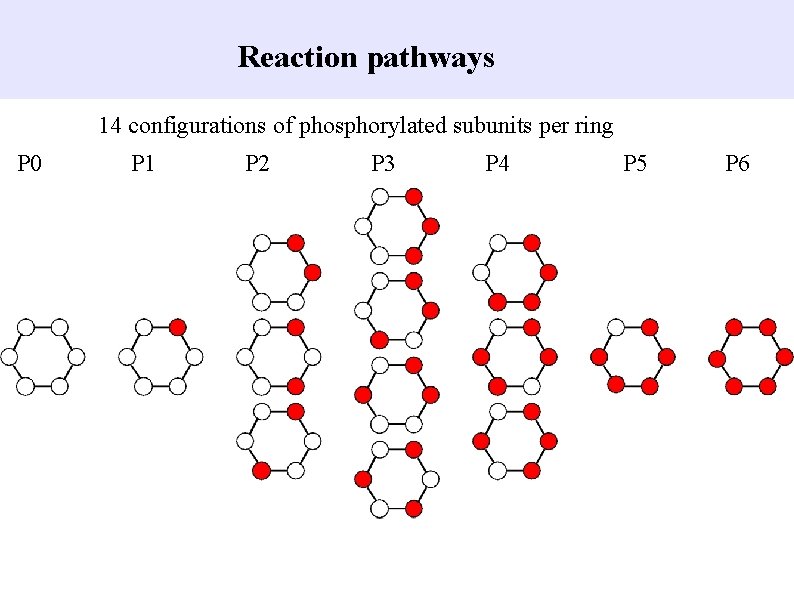
Reaction pathways 14 configurations of phosphorylated subunits per ring P 0 P 1 P 2 P 3 P 4 P 5 P 6

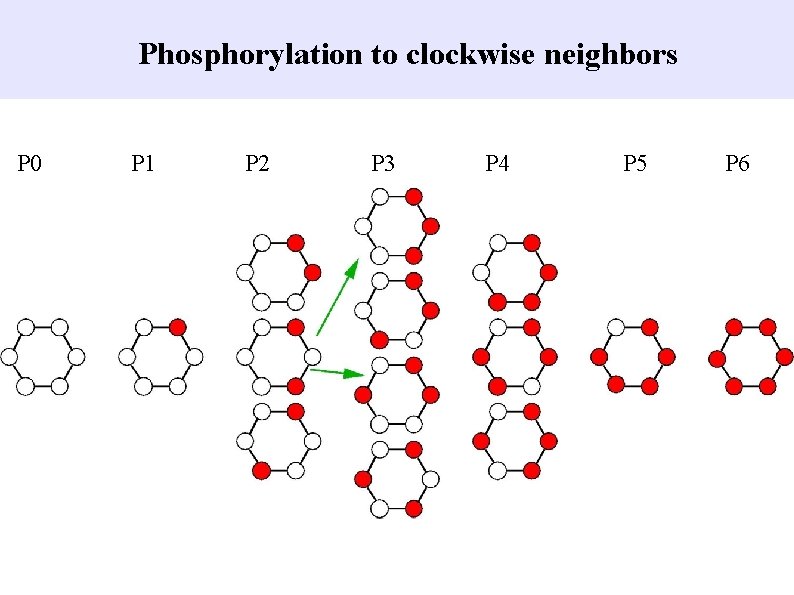
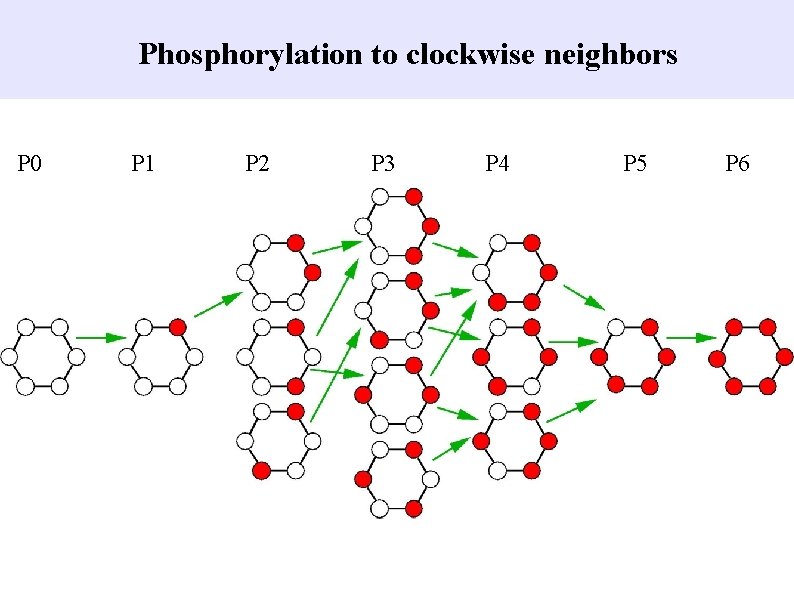
Phosphorylation to clockwise neighbors P 0 P 1 P 2 P 3 P 4 P 5 P 6

Phosphorylation to clockwise neighbors P 0 P 1 P 2 P 3 P 4 P 5 P 6

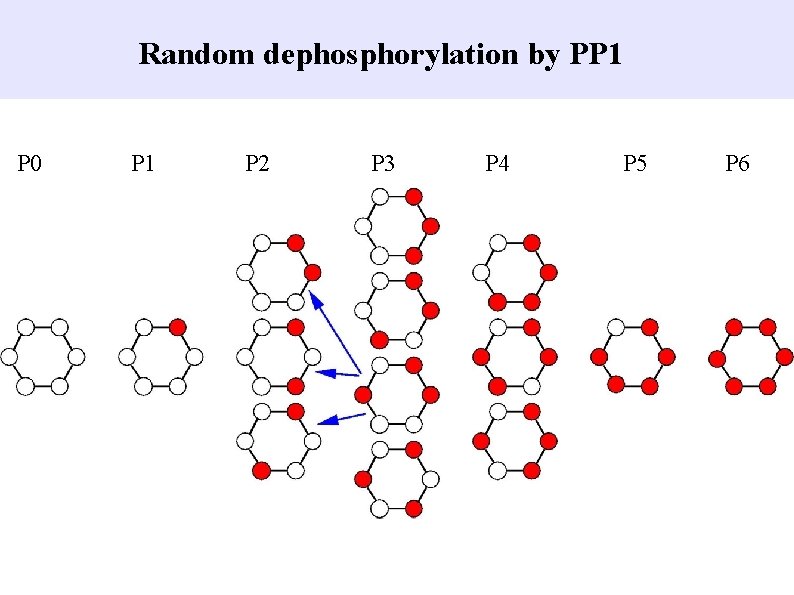
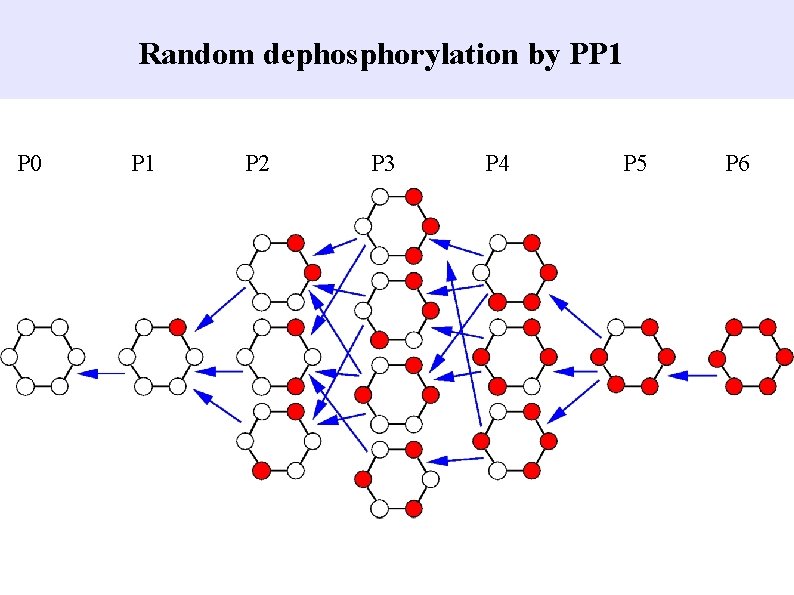
Random dephosphorylation by PP 1 P 0 P 1 P 2 P 3 P 4 P 5 P 6

Random dephosphorylation by PP 1 P 0 P 1 P 2 P 3 P 4 P 5 P 6

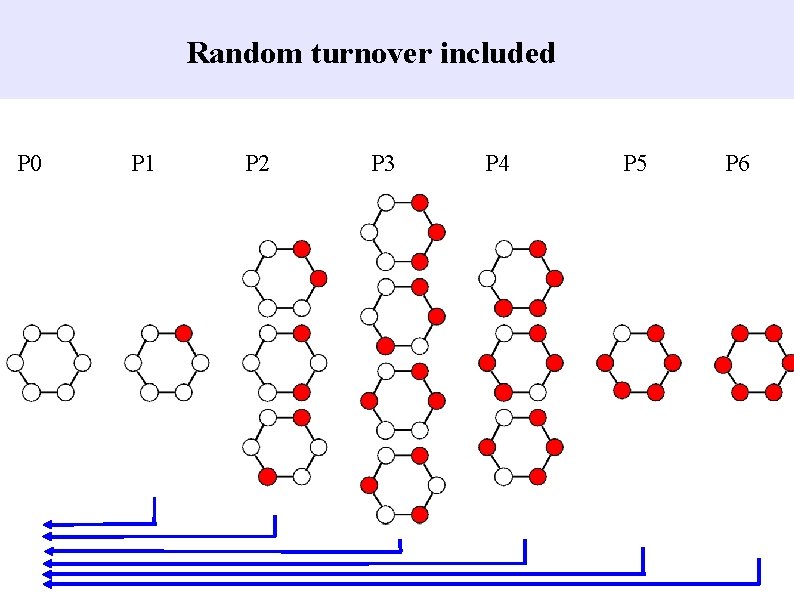
Random turnover included P 0 P 1 P 2 P 3 P 4 P 5 P 6

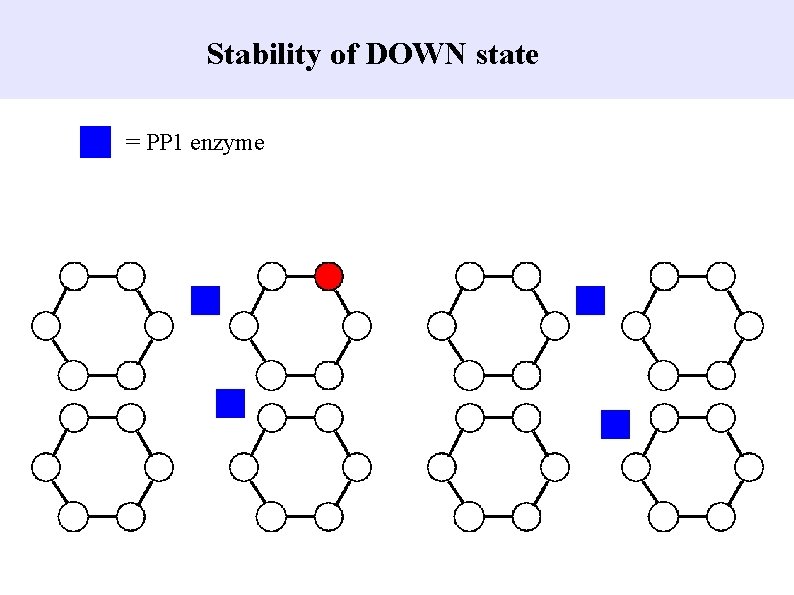
Stability of DOWN state = PP 1 enzyme

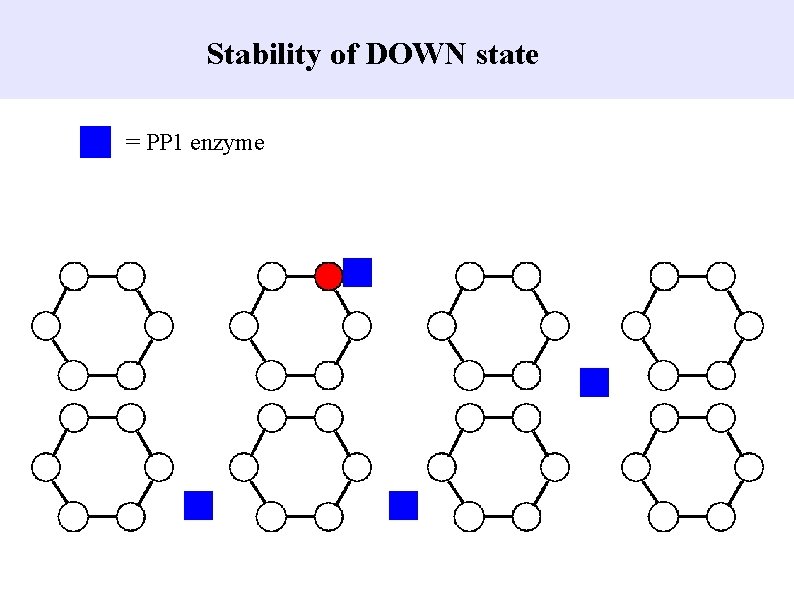
Stability of DOWN state = PP 1 enzyme

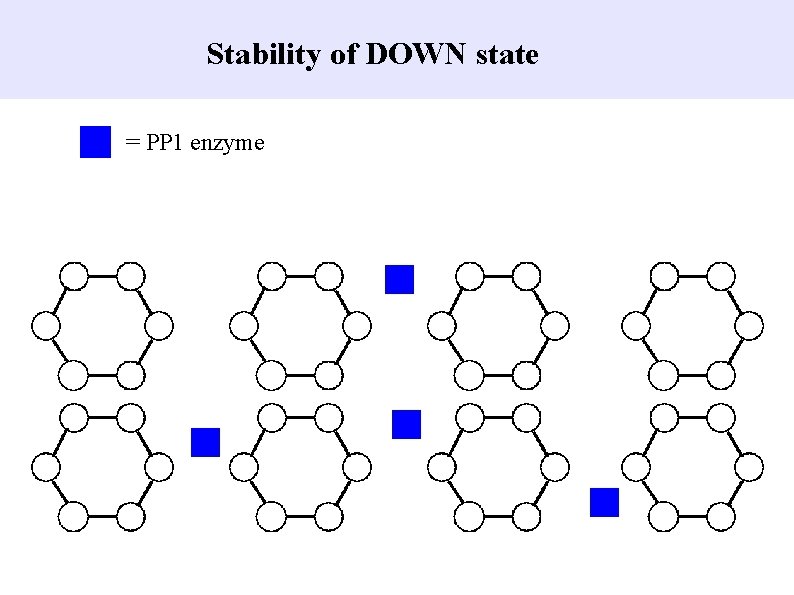
Stability of DOWN state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Protein turnover = PP 1 enzyme

Stability of UP state with turnover = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

Stability of UP state = PP 1 enzyme

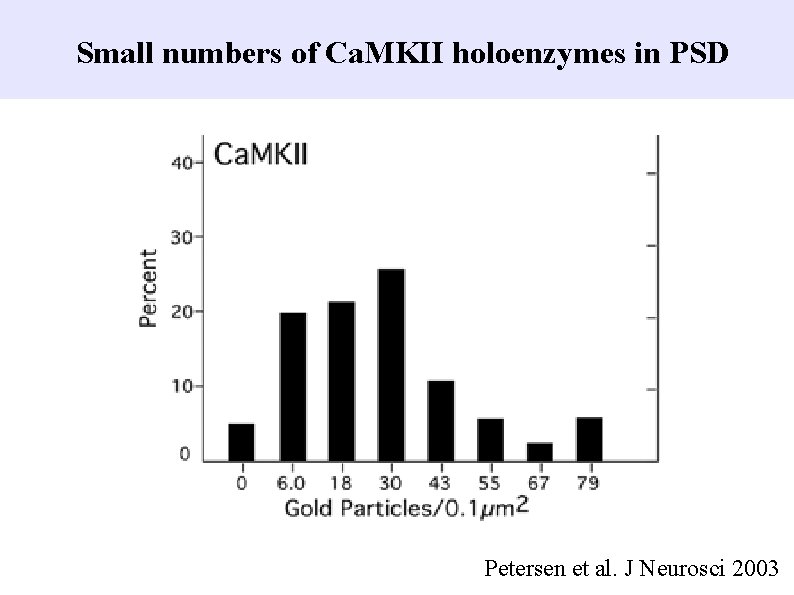
Small numbers of Ca. MKII holoenzymes in PSD Petersen et al. J Neurosci 2003

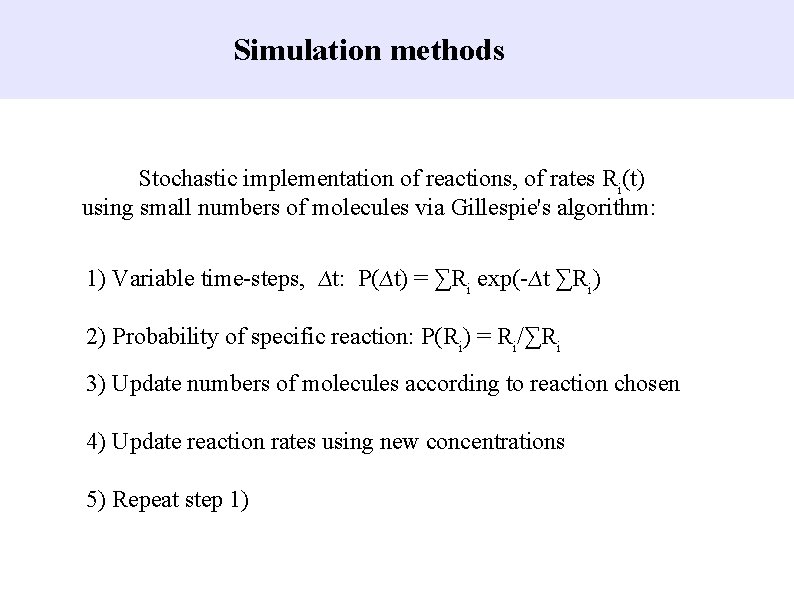
Simulation methods Stochastic implementation of reactions, of rates Ri(t) using small numbers of molecules via Gillespie's algorithm: 1) Variable time-steps, ∆t: P(∆t) = ∑Ri exp(-∆t ∑Ri) 2) Probability of specific reaction: P(Ri) = Ri/∑Ri 3) Update numbers of molecules according to reaction chosen 4) Update reaction rates using new concentrations 5) Repeat step 1)

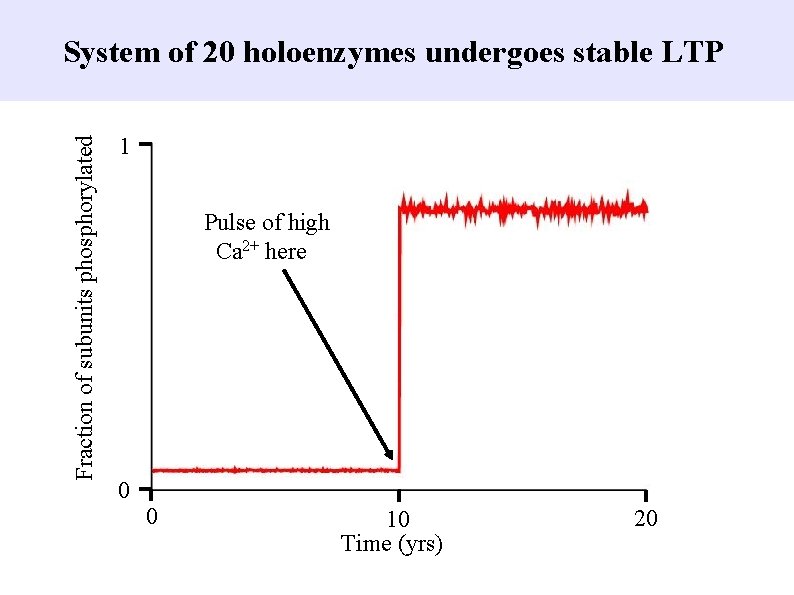
Fraction of subunits phosphorylated System of 20 holoenzymes undergoes stable LTP 1 Pulse of high Ca 2+ here 0 0 10 Time (yrs) 20

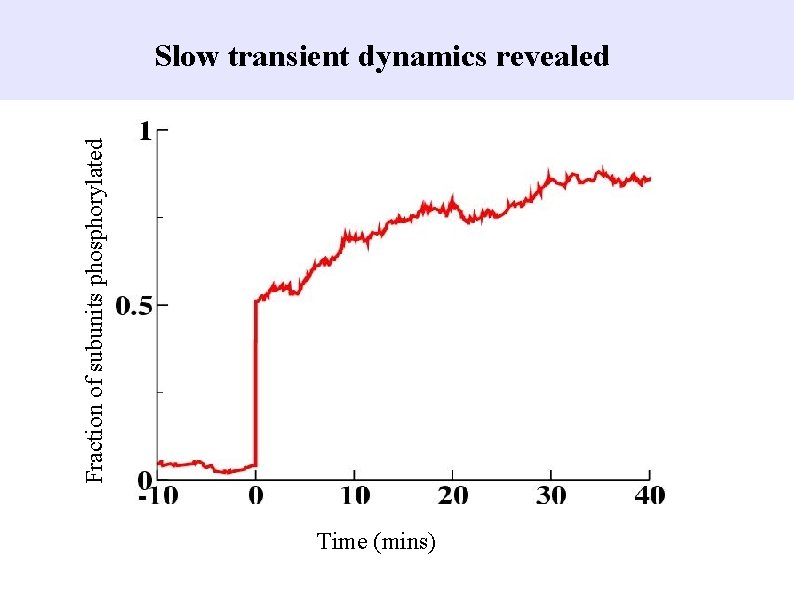
Fraction of subunits phosphorylated Slow transient dynamics revealed Time (mins)

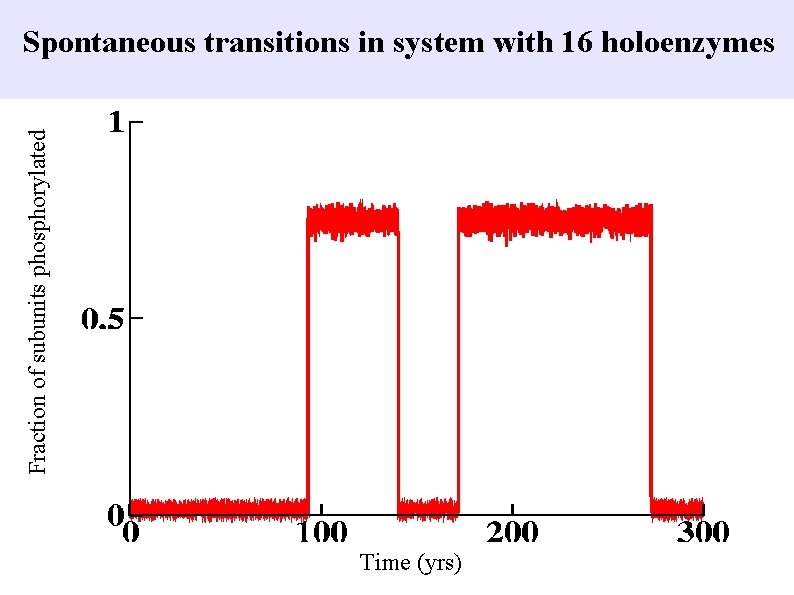
Fraction of subunits phosphorylated Spontaneous transitions in system with 16 holoenzymes Time (yrs)

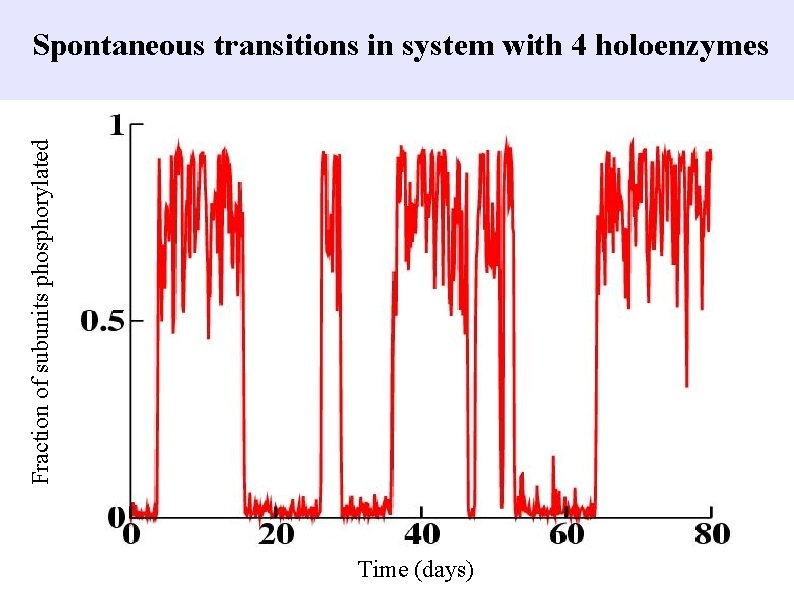
Fraction of subunits phosphorylated Spontaneous transitions in system with 4 holoenzymes Time (days)

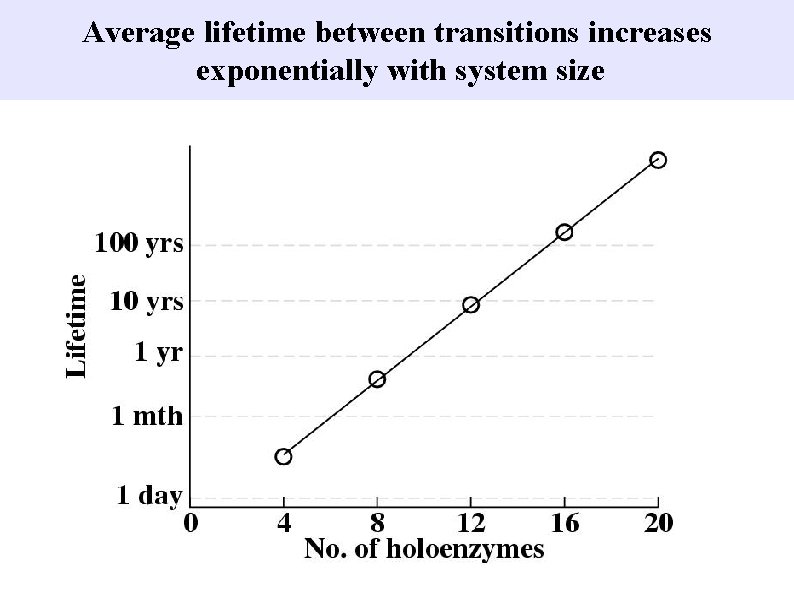
Average lifetime between transitions increases exponentially with system size

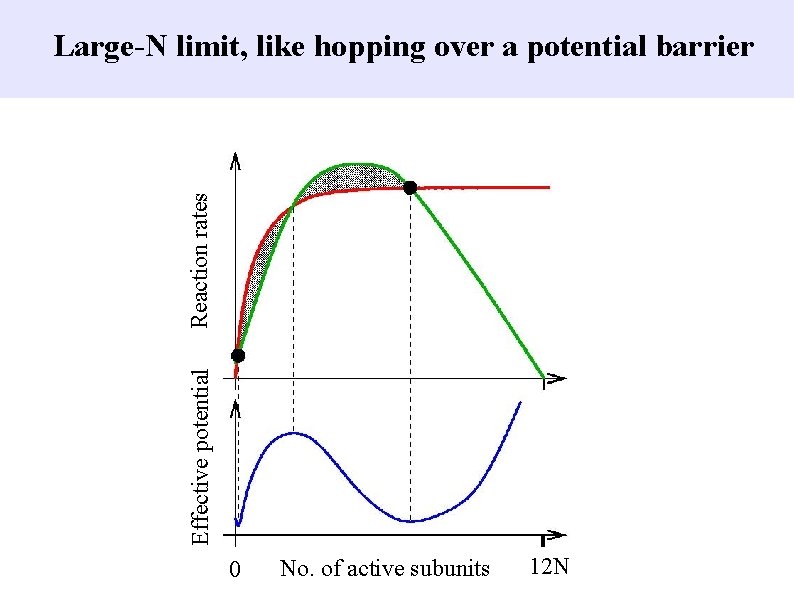
Effective potential Reaction rates Large-N limit, like hopping over a potential barrier 0 No. of active subunits 12 N

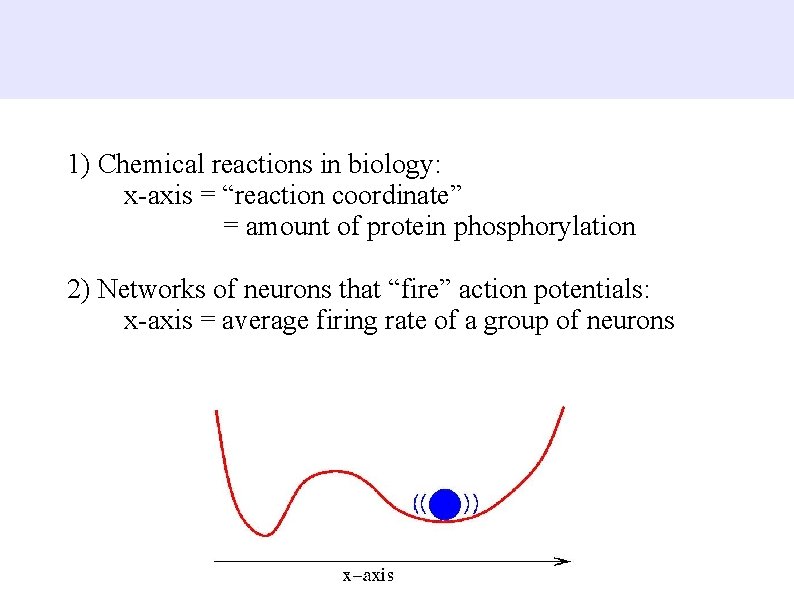
1) Chemical reactions in biology: x-axis = “reaction coordinate” = amount of protein phosphorylation 2) Networks of neurons that “fire” action potentials: x-axis = average firing rate of a group of neurons

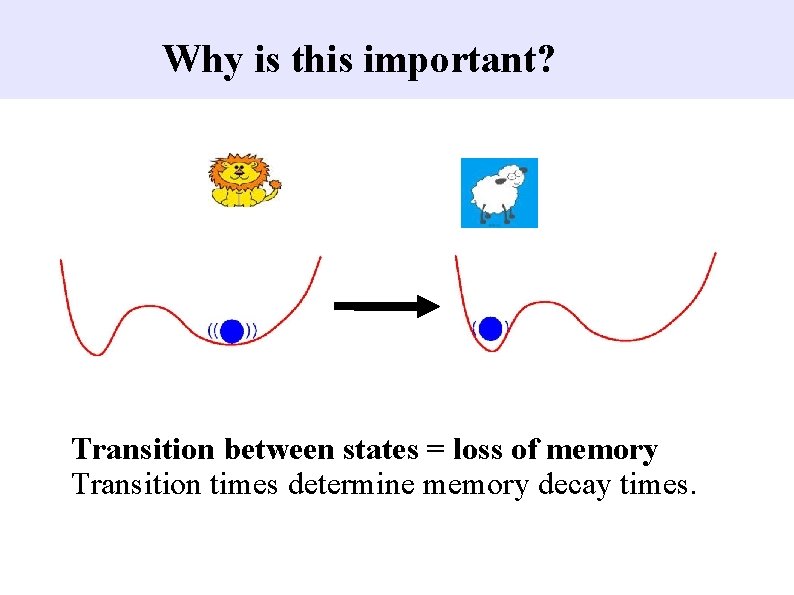
Why is this important? Transition between states = loss of memory Transition times determine memory decay times.

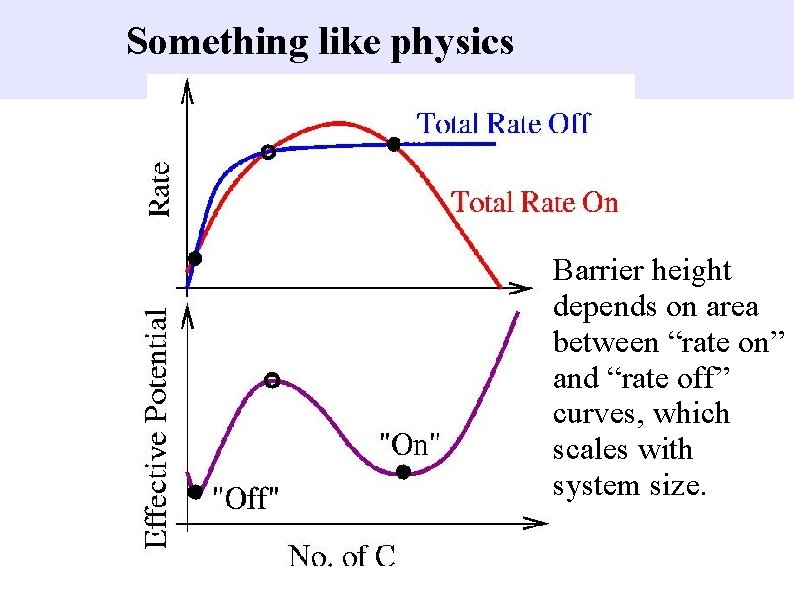
Something like physics Barrier height depends on area between “rate on” and “rate off” curves, which scales with system size.

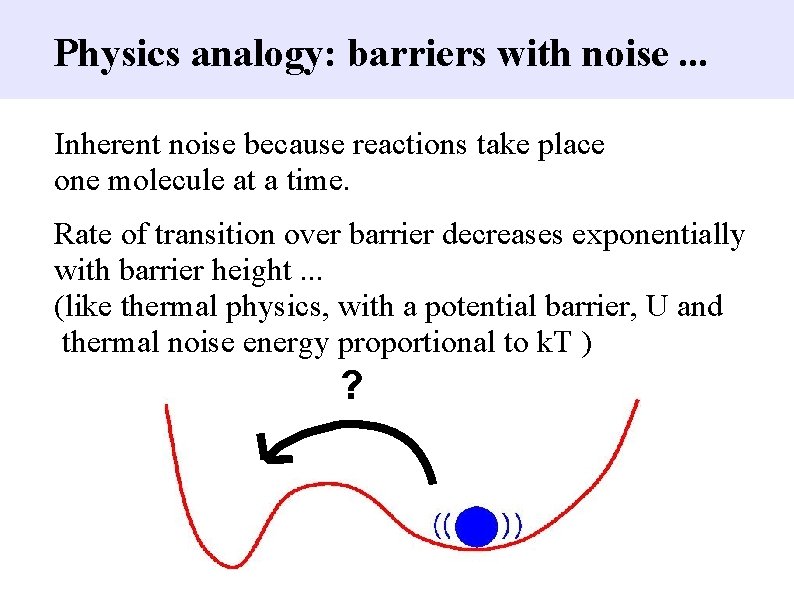
Physics analogy: barriers with noise. . . Inherent noise because reactions take place one molecule at a time. Rate of transition over barrier decreases exponentially with barrier height. . . (like thermal physics, with a potential barrier, U and thermal noise energy proportional to k. T ) ?

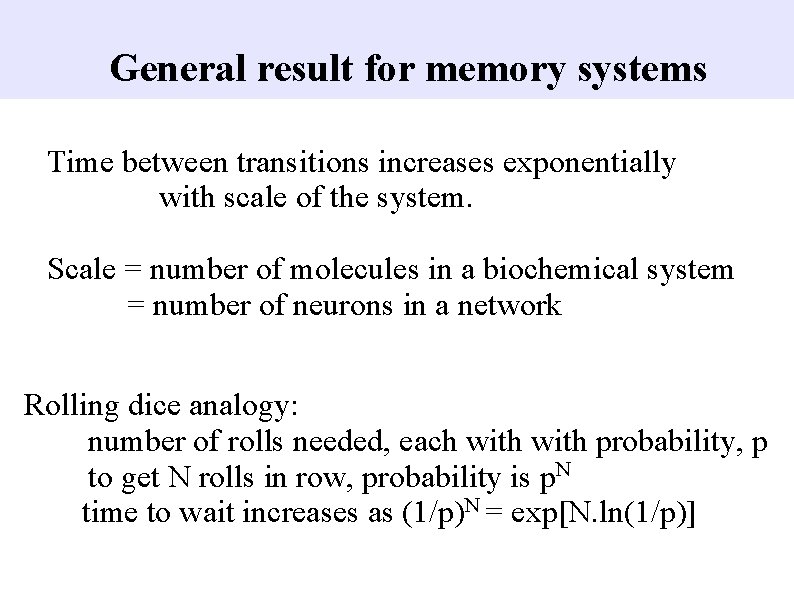
General result for memory systems Time between transitions increases exponentially with scale of the system. Scale = number of molecules in a biochemical system = number of neurons in a network Rolling dice analogy: number of rolls needed, each with probability, p to get N rolls in row, probability is p. N time to wait increases as (1/p)N = exp[N. ln(1/p)]

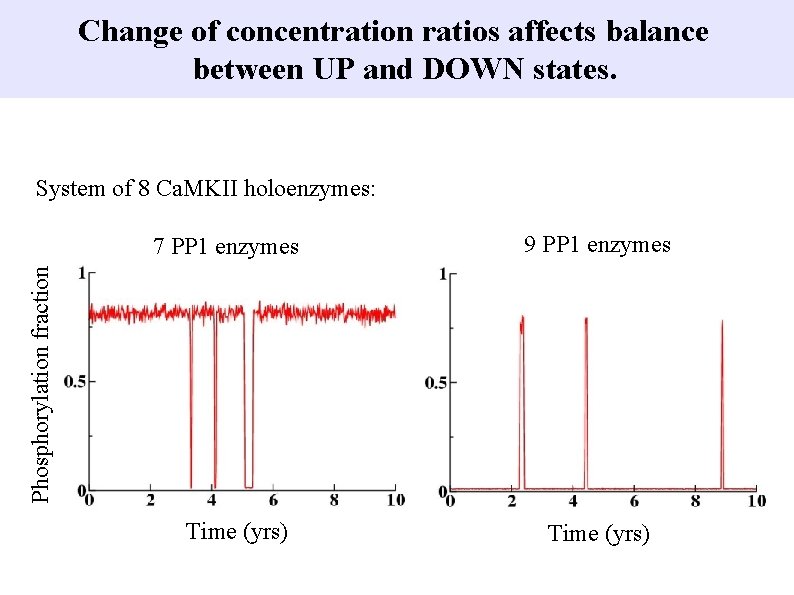
Change of concentration ratios affects balance between UP and DOWN states. System of 8 Ca. MKII holoenzymes: 9 PP 1 enzymes Phosphorylation fraction 7 PP 1 enzymes Time (yrs)

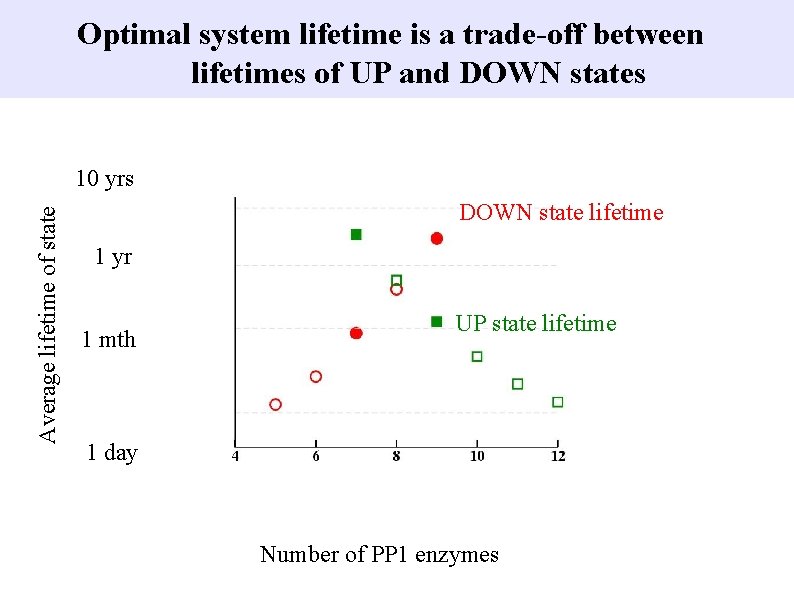
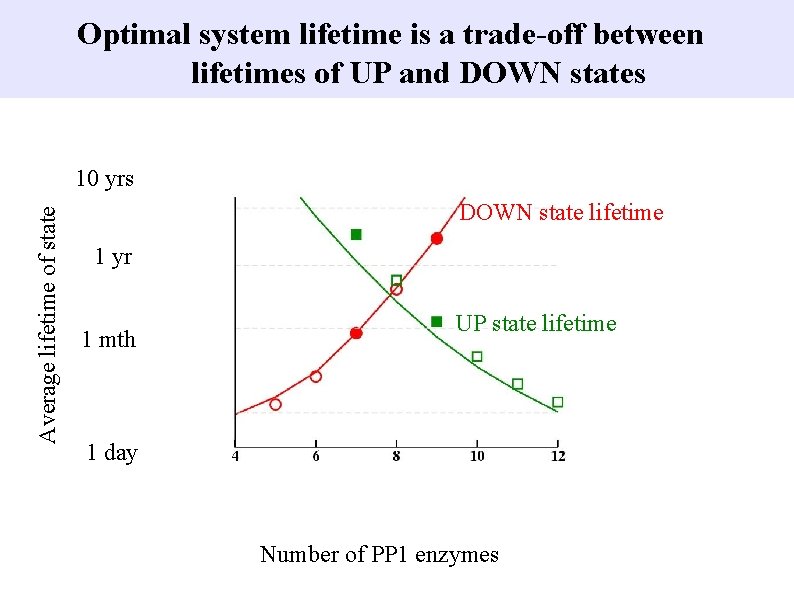
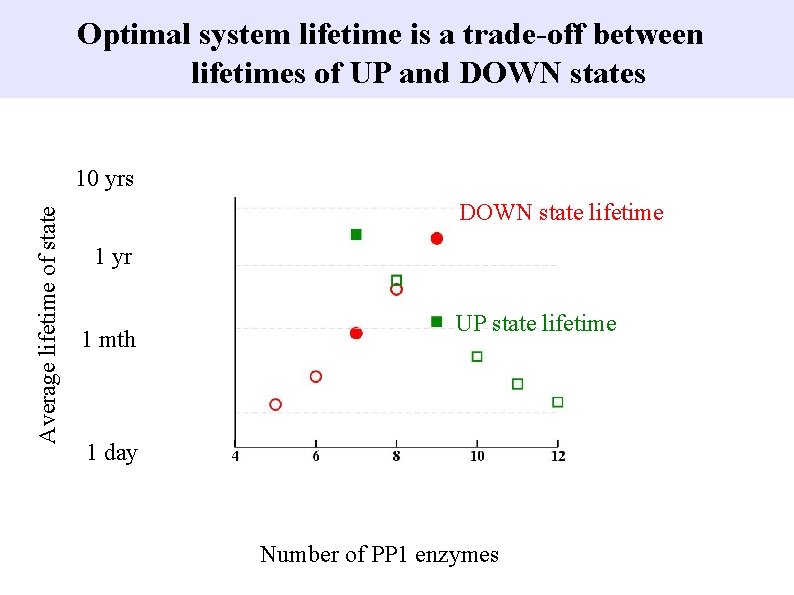
Optimal system lifetime is a trade-off between lifetimes of UP and DOWN states Average lifetime of state 10 yrs DOWN state lifetime 1 yr 1 mth UP state lifetime 1 day Number of PP 1 enzymes

Optimal system lifetime is a trade-off between lifetimes of UP and DOWN states Average lifetime of state 10 yrs DOWN state lifetime 1 yr 1 mth UP state lifetime 1 day Number of PP 1 enzymes

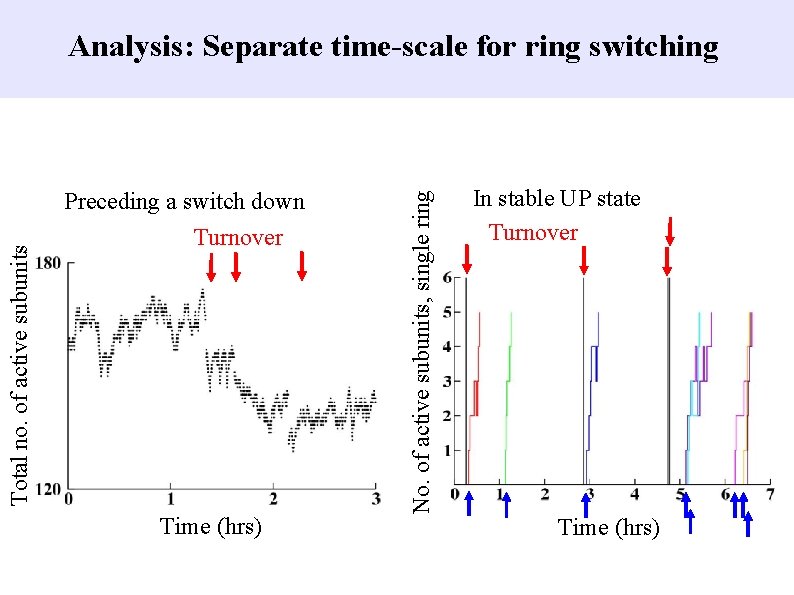
Preceding a switch down Turnover Time (hrs) No. of active subunits, single ring Total no. of active subunits Analysis: Separate time-scale for ring switching In stable UP state Turnover Time (hrs)


Analysis: Separate time-scale for ring switching Goal Rapid speed-up by converting system to 1 D and solving analytically. Method Essentially a mean-field theory. Justification Changes to and from P 0 (unphosphorylated state) are slow.

Analysis: Project system to 1 D 1) Number of rings “on” with any activation, n. 2) Assume average number, P, of subunits phosphorylated for all rings “on”. 3) Calculate reaction rates for one ring, assuming contibution of others is (n-1)P. 4) Calculate average time in configurations with these reaction rates. 5) Hence calculate new value of P. 6) Repeat Step 2 until convergence. 7) Calculate rate to switch “on”, r+n, and “off”, r-n. 8) Continue with new value of n.

Analysis: Project system to 1 D 1) Number of rings “on” with any activation, n. 2) Assume average number, P, of subunits phosphorylated for all rings “on”. 3) Calculate reaction rates for one ring, assuming contibution of others is (n-1)P. 4) Calculate average time in configurations with these reaction rates. 5) Hence calculate new value of P. 6) Repeat Step 2 until convergence. 7) Calculate rate to switch “on”, r+n, and “off”, r-n. 8) Continue with new value of n.

Analysis: Project system to 1 D 1) Number of rings “on” with any activation, n. 2) Assume average number, P, of subunits phosphorylated for all rings “on”. 3) Calculate reaction rates for one ring, assuming contibution of others is (n-1)P. 4) Calculate average time in configurations with these reaction rates. 5) Hence calculate new value of P. 6) Repeat Step 2 until convergence. 7) Calculate rate to switch “on”, r+n, and “off”, r-n. 8) Continue with new value of n.

Analysis: Project system to 1 D 1) Number of rings “on” with any activation, n. 2) Assume average number, P, of subunits phosphorylated for all rings “on”. 3) Calculate reaction rates for one ring, assuming contibution of others is (n-1)P. 4) Calculate average time in configurations with these reaction rates. 5) Hence calculate new value of P. 6) Repeat Step 2 until convergence. 7) Calculate rate to switch “on”, r+n, and “off”, r-n. 8) Continue with new value of n.

Analysis: Project system to 1 D 1) Number of rings “on” with any activation, n. 2) Assume average number, P, of subunits phosphorylated for all rings “on”. 3) Calculate reaction rates for one ring, assuming contibution of others is (n-1)P. 4) Calculate average time in configurations with these reaction rates. 5) Hence calculate new value of P. 6) Repeat Step 2 until convergence. 7) Calculate rate to switch “on”, r+n, and “off”, r-n. 8) Continue with new value of n.

Analysis: Project system to 1 D 1) Number of rings “on” with any activation, n. 2) Assume average number, P, of subunits phosphorylated for all rings “on”. 3) Calculate reaction rates for one ring, assuming contibution of others is (n-1)P. 4) Calculate average time in configurations with these reaction rates. 5) Hence calculate new value of P. 6) Repeat Step 2 until convergence. 7) Calculate rate to switch “on”, r+n, and “off”, r-n. 8) Continue with new value of n.

Analysis: Project system to 1 D 1) Number of rings “on” with any activation, n. 2) Assume average number, P, of subunits phosphorylated for all rings “on”. 3) Calculate reaction rates for one ring, assuming contibution of others is (n-1)P. 4) Calculate average time in configurations with these reaction rates. 5) Hence calculate new value of P. 6) Repeat Step 2 until convergence. 7) Calculate rate to switch “on”, r+n, and “off”, r-n. 8) Continue with new value of n.

Analysis: Project system to 1 D 1) Number of rings “on” with any activation, n. 2) Assume average number, P, of subunits phosphorylated for all rings “on”. 3) Calculate reaction rates for one ring, assuming contibution of others is (n-1)P. 4) Calculate average time in configurations with these reaction rates. 5) Hence calculate new value of P. 6) Repeat Step 2 until convergence. 7) Calculate rate to switch “on”, r+n, and “off”, r-n. 8) Continue with new value of n.

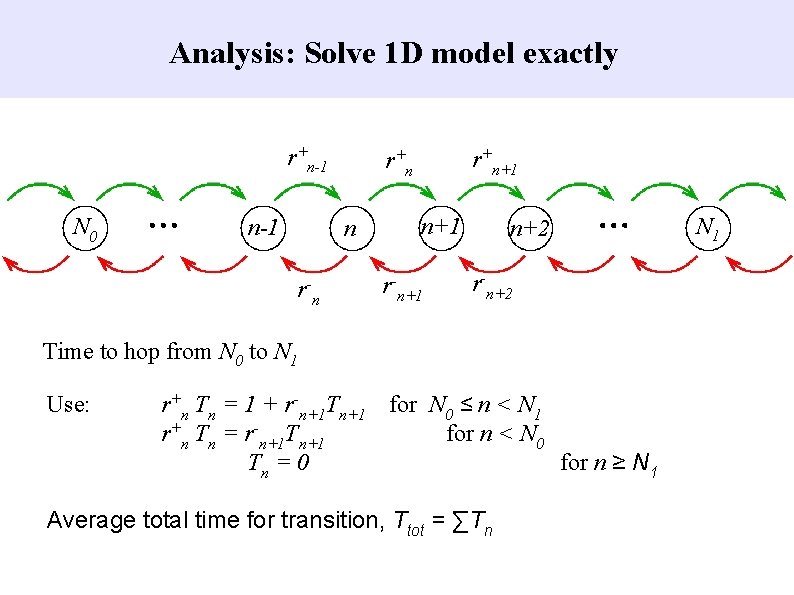
Analysis: Solve 1 D model exactly r+n-1 N 0 n-1 n - rn r+n+1 r+ n n+1 r-n+1 n+2 r-n+2 Time to hop from N 0 to N 1 Use: r+n Tn = 1 + r-n+1 Tn+1 for N 0 ≤ n < N 1 r+n Tn = r-n+1 Tn+1 for n < N 0 Tn = 0 for n ≥ N 1 Average total time for transition, Ttot = ∑Tn N 1

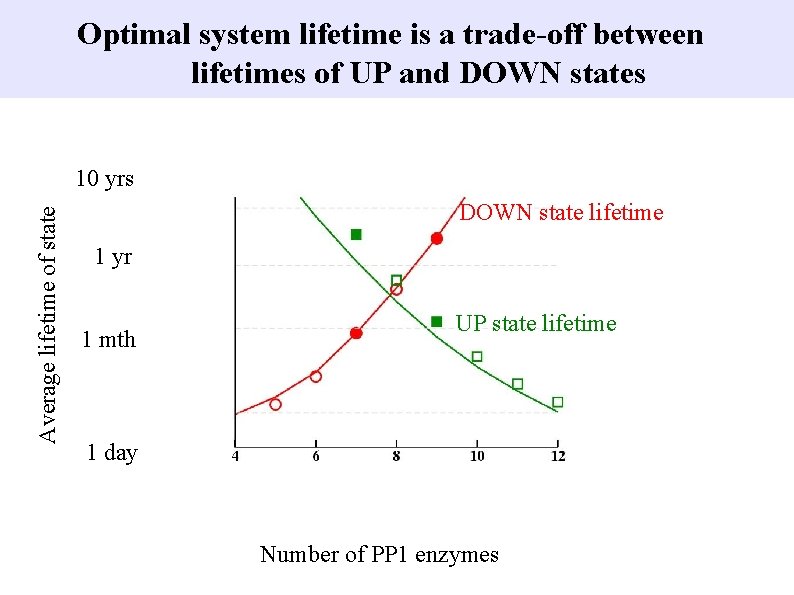
Optimal system lifetime is a trade-off between lifetimes of UP and DOWN states Average lifetime of state 10 yrs DOWN state lifetime 1 yr 1 mth UP state lifetime 1 day Number of PP 1 enzymes

Optimal system lifetime is a trade-off between lifetimes of UP and DOWN states Average lifetime of state 10 yrs DOWN state lifetime 1 yr 1 mth UP state lifetime 1 day Number of PP 1 enzymes