Molecular Interactions Dr Henry Curran NUI Galway Physical





















- Slides: 78

Molecular Interactions Dr. Henry Curran NUI Galway Physical Chemistry (CH 313) Atkins and de Paula, Chapter 17 1

Background Atoms and molecules with complete valence shells can still interact with one another even though all of their valences are satisfied. They attract one another over a range of several atomic diameters and repel one another when pressed together. 2

Molecular interactions account for: – condensation of gases to liquids – structures of molecular solids – structural organisation of biological macromolecules as they pin molecular building blocks (polypeptides, polynucleotides, and lipids) together in the arrangement essential to their proper physiological function. 3

van der Waals interactions • Interaction between partial charges in polar molecules • Electric dipole moments or charge distribution • Interactions between dipoles • Induced dipole moments • Dispersion interactions – Interaction between species with neither a net charge nor a permanent electric dipole moment (e. g. two Xe atoms) 4

The total interaction • Hydrogen bonding • The hydrophobic effect • Modelling the total interaction • Molecules in motion 5

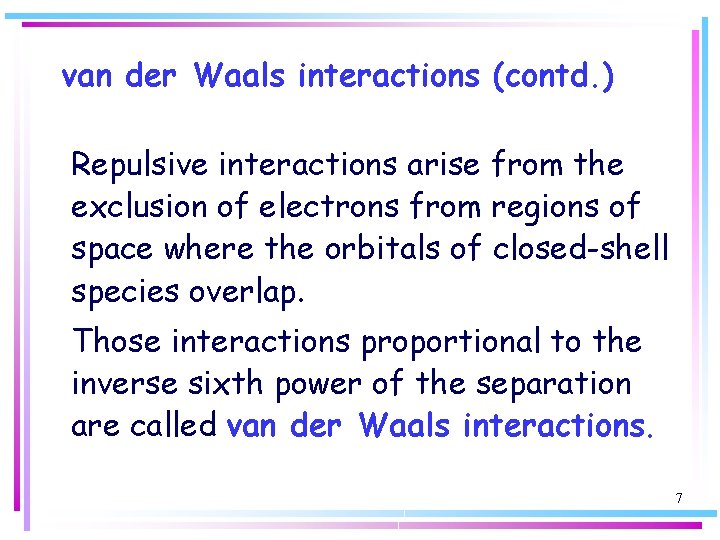
van der Waals interactions Interactions between molecules include the attractive and repulsive interactions between the partial electric charges of polar molecules and the repulsive interactions that prevent the complete collapse of matter to densities as high as those characteristic of atomic nuclei. 6

van der Waals interactions (contd. ) Repulsive interactions arise from the exclusion of electrons from regions of space where the orbitals of closed-shell species overlap. Those interactions proportional to the inverse sixth power of the separation are called van der Waals interactions. 7

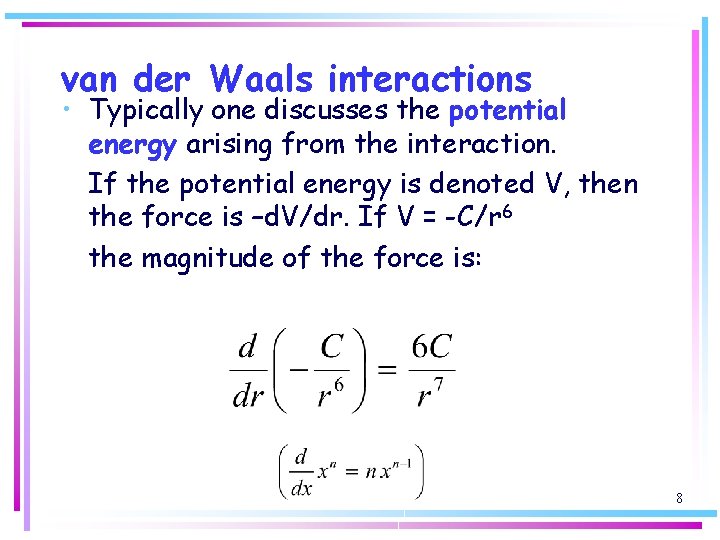
van der Waals interactions • Typically one discusses the potential energy arising from the interaction. If the potential energy is denoted V, then the force is –d. V/dr. If V = -C/r 6 the magnitude of the force is: 8

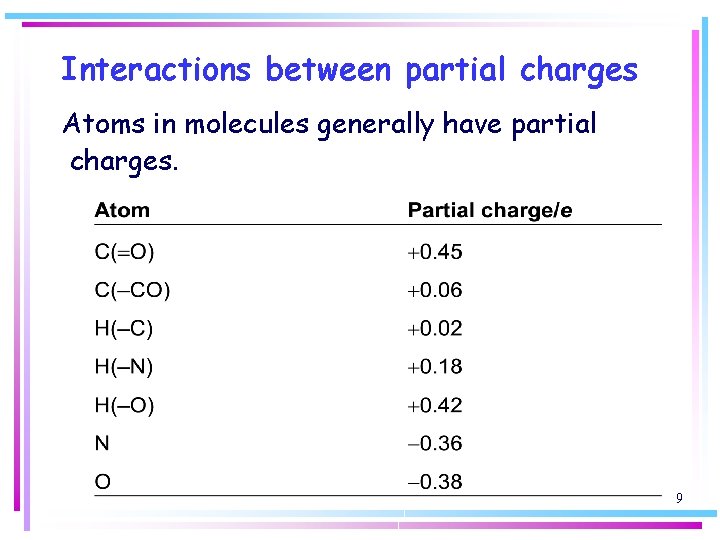
Interactions between partial charges Atoms in molecules generally have partial charges. 9

Interactions between partial charges • If these charges were separated by a vacuum, they would attract or repel one another according to Coulomb’s Law: where q 1 and q 2 are the partial charges and r is their separation 10

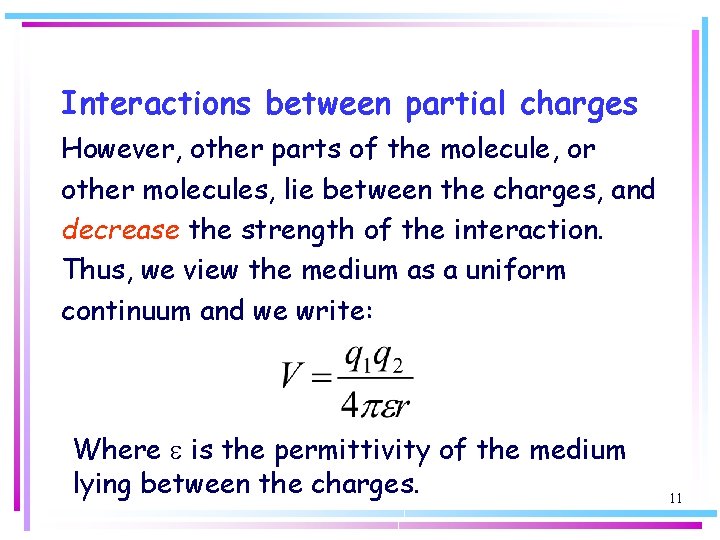
Interactions between partial charges However, other parts of the molecule, or other molecules, lie between the charges, and decrease the strength of the interaction. Thus, we view the medium as a uniform continuum and we write: Where e is the permittivity of the medium lying between the charges. 11

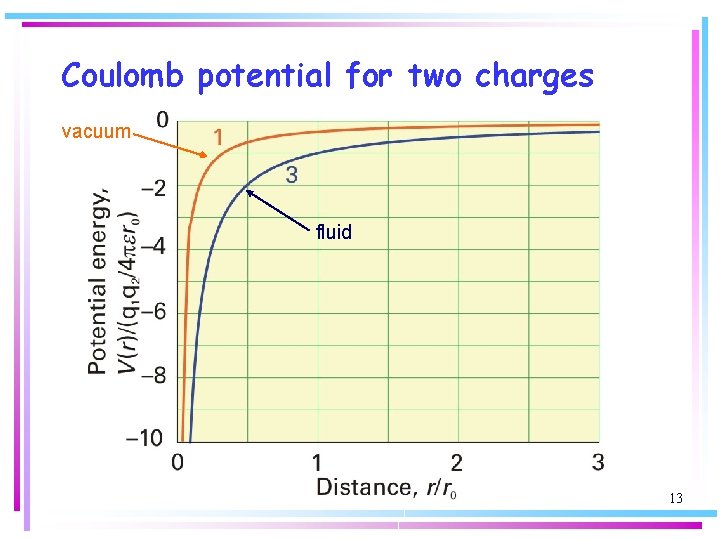
• The permittivity is usually expressed as a multiple of the vacuum permittivity by writing e = ere 0, where er is the relative permittivity (dielectric constant). The effect of the medium can be very large, for water at 250 C, er = 78. The PE of two charges separated by bulk water is reduced by nearly two orders of magnitude compared to that if the charges were separated by a vacuum. 12

Coulomb potential for two charges vacuum fluid 13

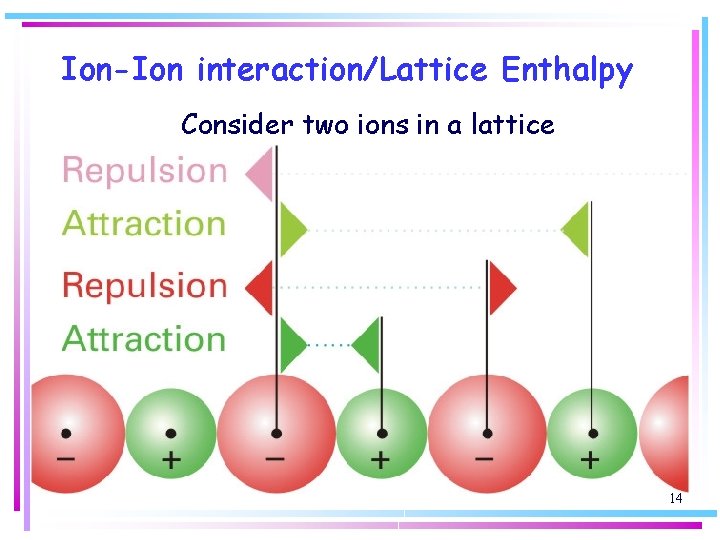
Ion-Ion interaction/Lattice Enthalpy Consider two ions in a lattice 14

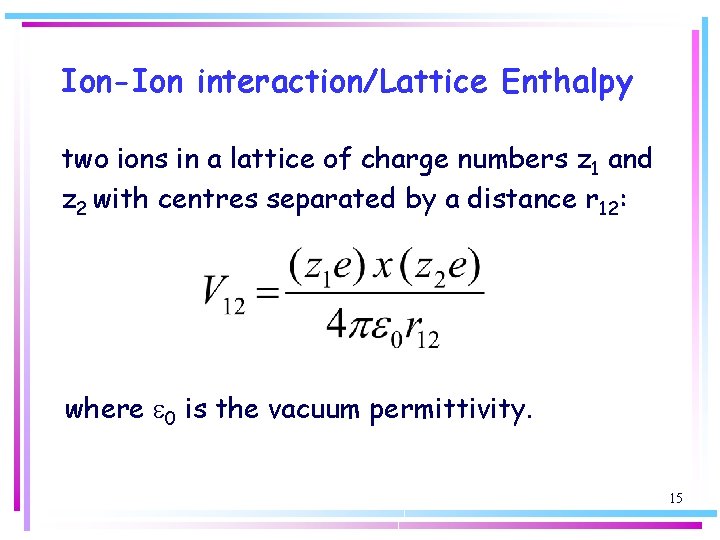
Ion-Ion interaction/Lattice Enthalpy two ions in a lattice of charge numbers z 1 and z 2 with centres separated by a distance r 12: where e 0 is the vacuum permittivity. 15

Ion-Ion interaction/Lattice Enthalpy To calculate the total potential energy of all the ions in the crystal, we have to sum this expression over all the ions. Nearest neighbours attract, while second-nearest repel and contribute a slightly weaker negative term to the overall energy. Overall, there is a net attraction resulting in a negative contribution to the energy of the solid. 16

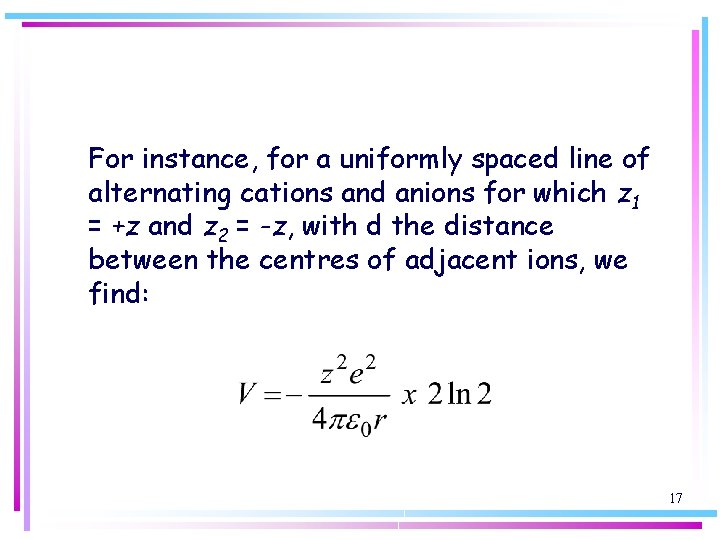
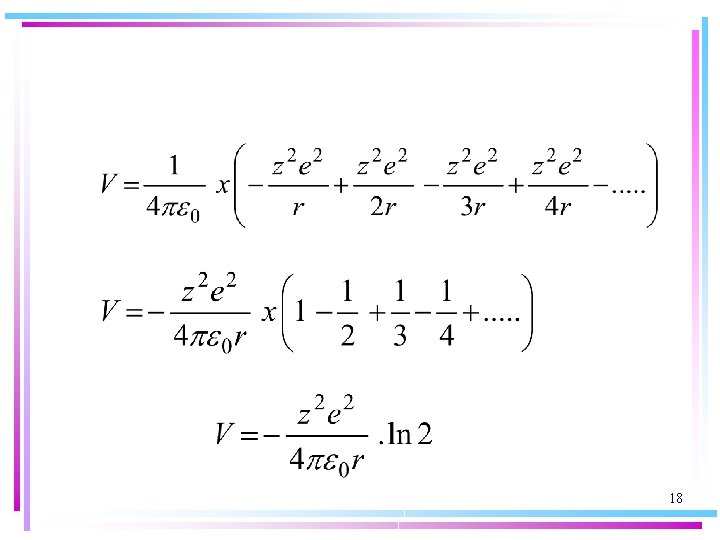
For instance, for a uniformly spaced line of alternating cations and anions for which z 1 = +z and z 2 = -z, with d the distance between the centres of adjacent ions, we find: 17

18

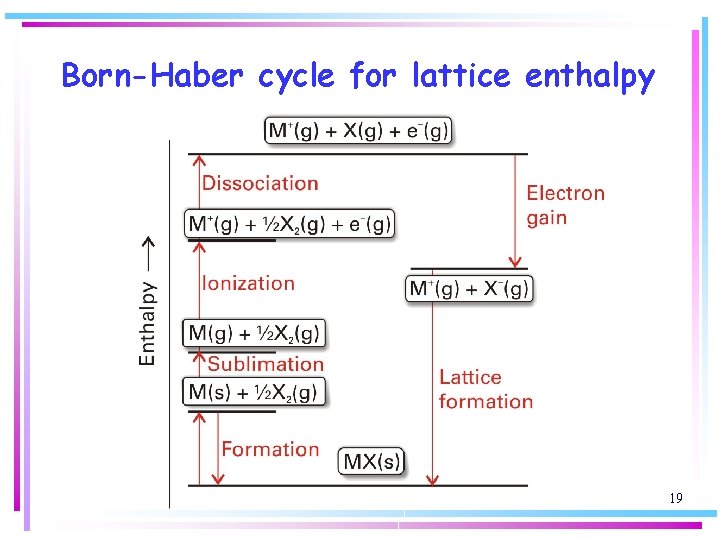
Born-Haber cycle for lattice enthalpy 19

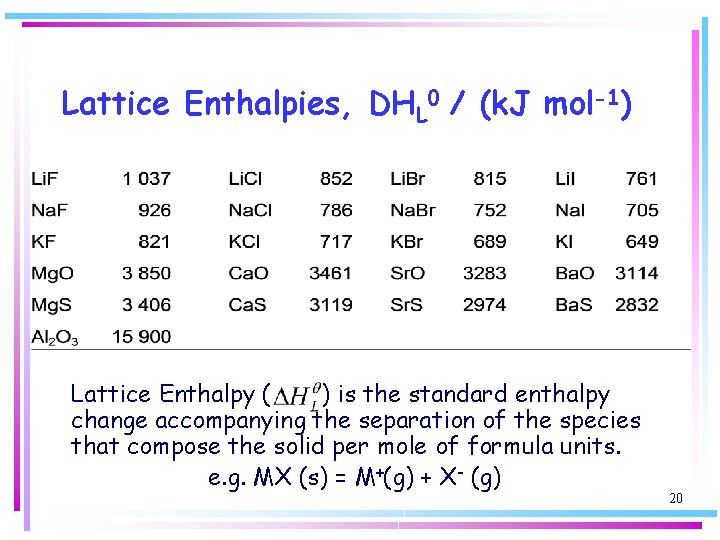
Lattice Enthalpies, DHL 0 / (k. J mol-1) Lattice Enthalpy ( ) is the standard enthalpy change accompanying the separation of the species that compose the solid per mole of formula units. e. g. MX (s) = M+(g) + X- (g) 20

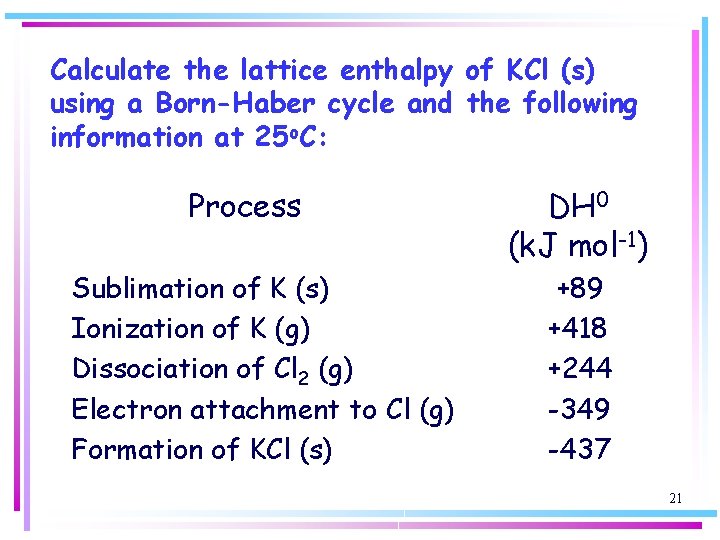
Calculate the lattice enthalpy of KCl (s) using a Born-Haber cycle and the following information at 25 o. C: Process Sublimation of K (s) Ionization of K (g) Dissociation of Cl 2 (g) Electron attachment to Cl (g) Formation of KCl (s) DH 0 (k. J mol-1) +89 +418 +244 -349 -437 21

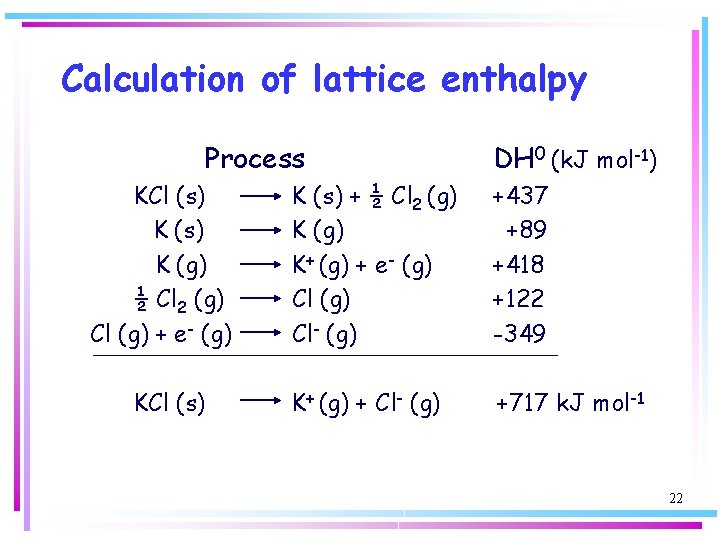
Calculation of lattice enthalpy Process KCl (s) K (g) ½ Cl 2 (g) Cl (g) + e- (g) KCl (s) DH 0 (k. J mol-1) K (s) + ½ Cl 2 (g) K+ (g) + e- (g) Cl- (g) +437 +89 +418 +122 -349 K+ (g) + Cl- (g) +717 k. J mol-1 22

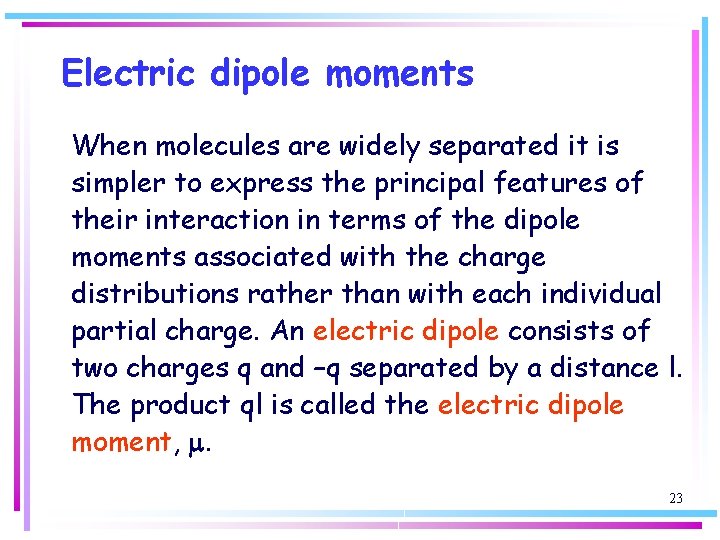
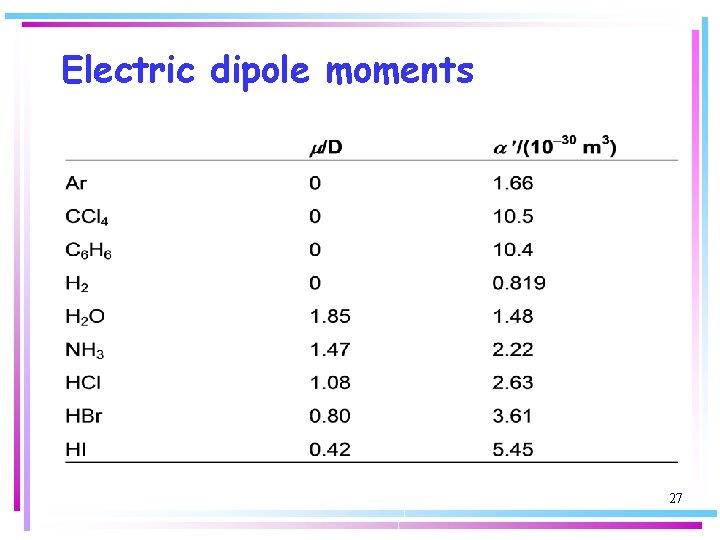
Electric dipole moments When molecules are widely separated it is simpler to express the principal features of their interaction in terms of the dipole moments associated with the charge distributions rather than with each individual partial charge. An electric dipole consists of two charges q and –q separated by a distance l. The product ql is called the electric dipole moment, m. 23

Electric dipole moments We represent dipole moments by an arrow with a length proportional to m and pointing from the negative charge to the positive charge: m d+ d- Because a dipole moment is the product of a charge and a length the SI unit of dipole moment is the coulomb-metre (C m) 24

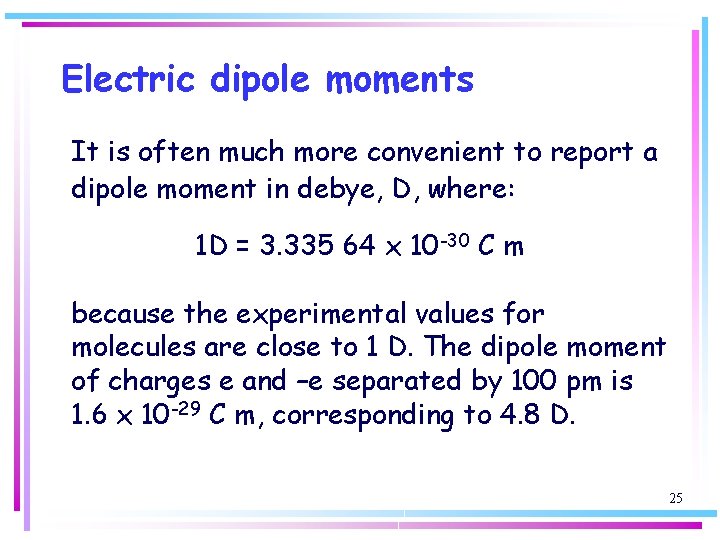
Electric dipole moments It is often much more convenient to report a dipole moment in debye, D, where: 1 D = 3. 335 64 x 10 -30 C m because the experimental values for molecules are close to 1 D. The dipole moment of charges e and –e separated by 100 pm is 1. 6 x 10 -29 C m, corresponding to 4. 8 D. 25

Electric dipole moments: diatomic molecules A polar molecule has a permanent electric dipole moment arising from the partial charges on its atoms. All hetero-nuclear diatomic molecules are polar because the difference in electronegativities of their two atoms results in non-zero partial charges. 26

Electric dipole moments 27

Electric dipole moments: diatomic molecules More electronegative atom is usually the negative end of the dipole. There are exceptions, particularly when anti-bonding orbitals are occupied. – CO dipole moment is small (0. 12 D) but negative end is on C atom. Anti -bonding orbitals are occupied in CO and electrons in anti-bonding orbitals are closer to the less electronegative atom, contributing a negative partial charge to that atom. If this contribution is larger than the opposite contribution from the electrons in bonding orbitals, there is a small negative charge on the less electronegative atom. 28

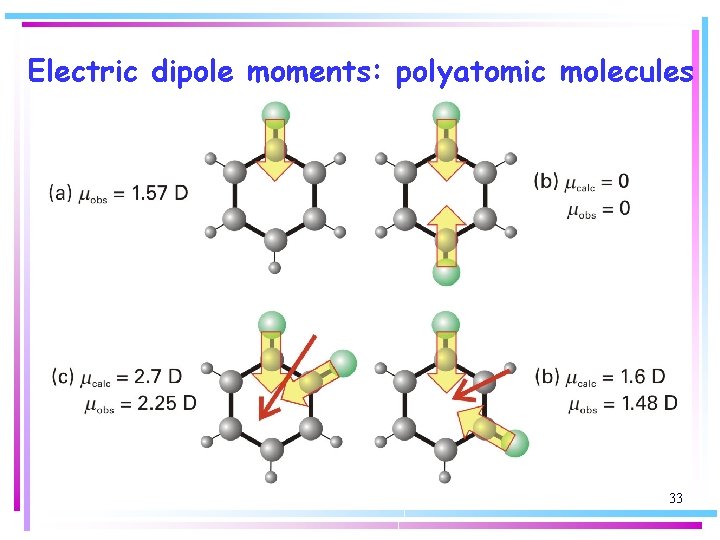
Electric dipole moments: polyatomic molecules Molecular symmetry is of the greatest importance in deciding whether a polyatomic molecule is polar or not. Homo-nuclear polyatomic molecules may be polar if they have low symmetry – in ozone, dipole moments associated with each bond make an angle with one another and do not cancel. d+ d+ m m d d Ozone, O 3 29

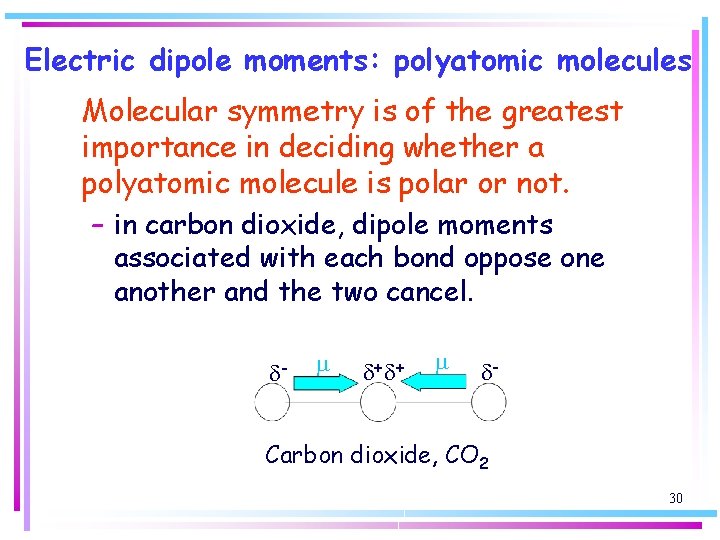
Electric dipole moments: polyatomic molecules Molecular symmetry is of the greatest importance in deciding whether a polyatomic molecule is polar or not. – in carbon dioxide, dipole moments associated with each bond oppose one another and the two cancel. d- m d+ d+ m d- Carbon dioxide, CO 2 30

Electric dipole moments: polyatomic molecules It is possible to resolve the dipole moment of a polyatomic molecule into contributions from various groups of atoms in the molecule and the direction in which each of these contributions lie. 31

Electric dipole moments: polyatomic molecules 1, 2 -dichlorobenzene: two chlorobenzene dipole moments arranged at 60 o to each other. Using vector addition the resultant dipole moment (mres) of two dipole moments m 1 and m 2 that make an angle q with one another is approximately: mres m 1 q m 2 32

Electric dipole moments: polyatomic molecules 33

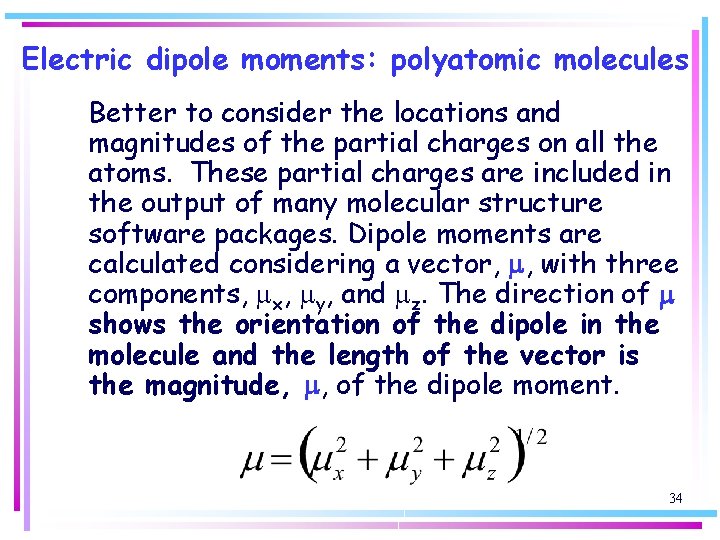
Electric dipole moments: polyatomic molecules Better to consider the locations and magnitudes of the partial charges on all the atoms. These partial charges are included in the output of many molecular structure software packages. Dipole moments are calculated considering a vector, m, with three components, mx, my, and mz. The direction of m shows the orientation of the dipole in the molecule and the length of the vector is the magnitude, m, of the dipole moment. 34

Electric dipole moments: polyatomic molecules To calculate the x-component we need to know the partial charge on each atom and the atom’s x-coordinate relative to a point in the molecule and from the sum: mz where q. J is the partial charge of atom J, x. J is the x coordinate of atom J, and the sum is over all atoms in molecule my m mx 35

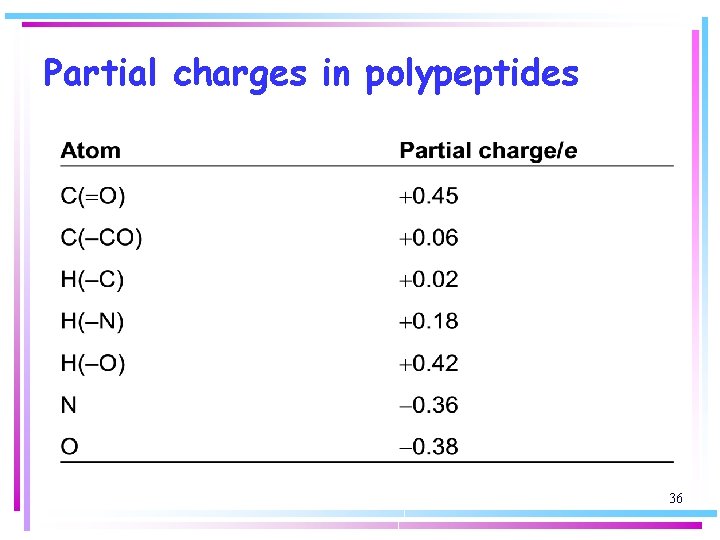
Partial charges in polypeptides 36

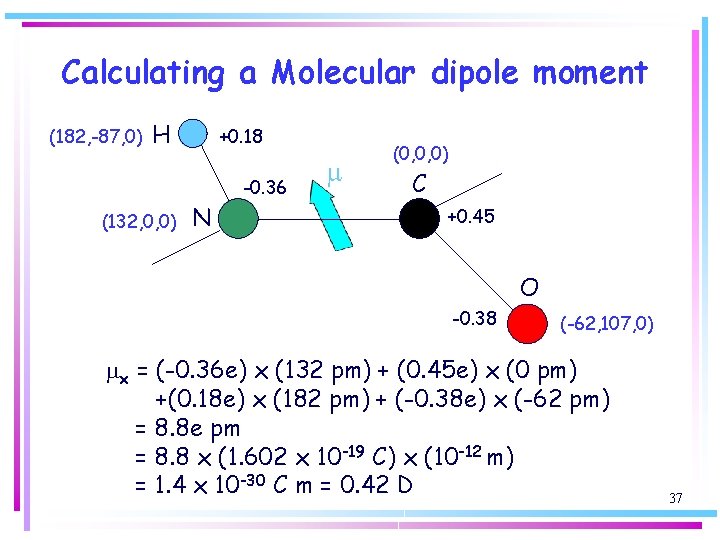
Calculating a Molecular dipole moment (182, -87, 0) H +0. 18 -0. 36 (132, 0, 0) N m (0, 0, 0) C +0. 45 O -0. 38 (-62, 107, 0) mx = (-0. 36 e) x (132 pm) + (0. 45 e) x (0 pm) +(0. 18 e) x (182 pm) + (-0. 38 e) x (-62 pm) = 8. 8 e pm = 8. 8 x (1. 602 x 10 -19 C) x (10 -12 m) = 1. 4 x 10 -30 C m = 0. 42 D 37

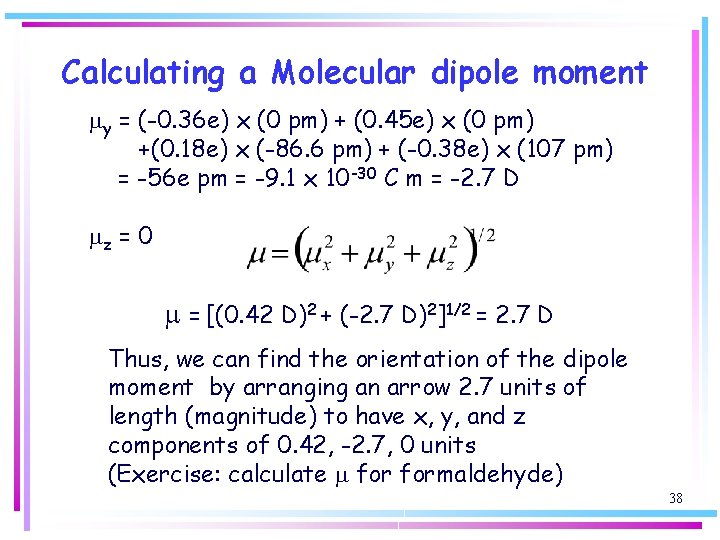
Calculating a Molecular dipole moment my = (-0. 36 e) x (0 pm) + (0. 45 e) x (0 pm) +(0. 18 e) x (-86. 6 pm) + (-0. 38 e) x (107 pm) = -56 e pm = -9. 1 x 10 -30 C m = -2. 7 D mz = 0 m = [(0. 42 D)2 + (-2. 7 D)2]1/2 = 2. 7 D Thus, we can find the orientation of the dipole moment by arranging an arrow 2. 7 units of length (magnitude) to have x, y, and z components of 0. 42, -2. 7, 0 units (Exercise: calculate m formaldehyde) 38

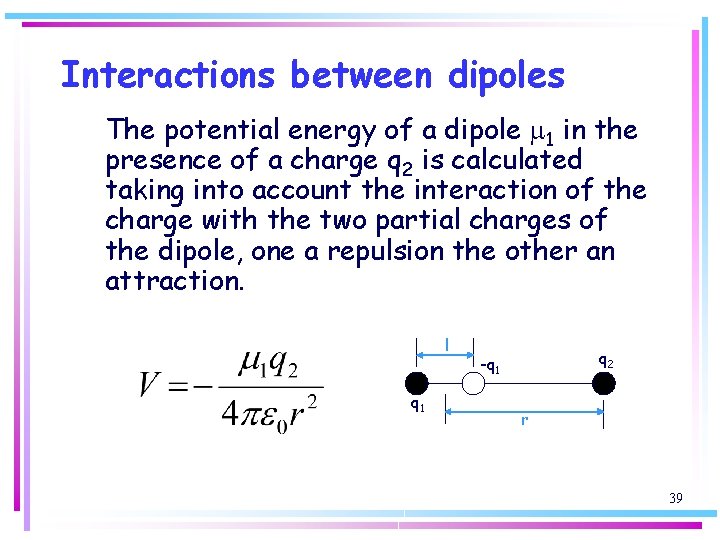
Interactions between dipoles The potential energy of a dipole m 1 in the presence of a charge q 2 is calculated taking into account the interaction of the charge with the two partial charges of the dipole, one a repulsion the other an attraction. l q 1 q 2 -q 1 r 39

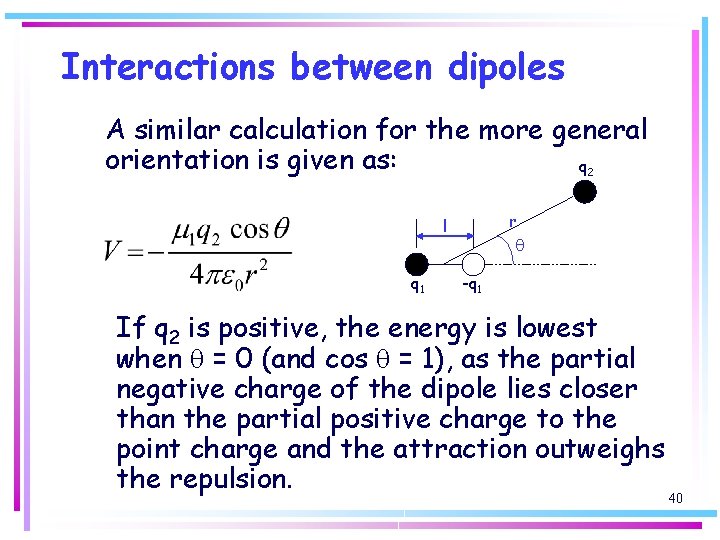
Interactions between dipoles A similar calculation for the more general orientation is given as: q 2 r l q 1 q -q 1 If q 2 is positive, the energy is lowest when q = 0 (and cos q = 1), as the partial negative charge of the dipole lies closer than the partial positive charge to the point charge and the attraction outweighs the repulsion. 40

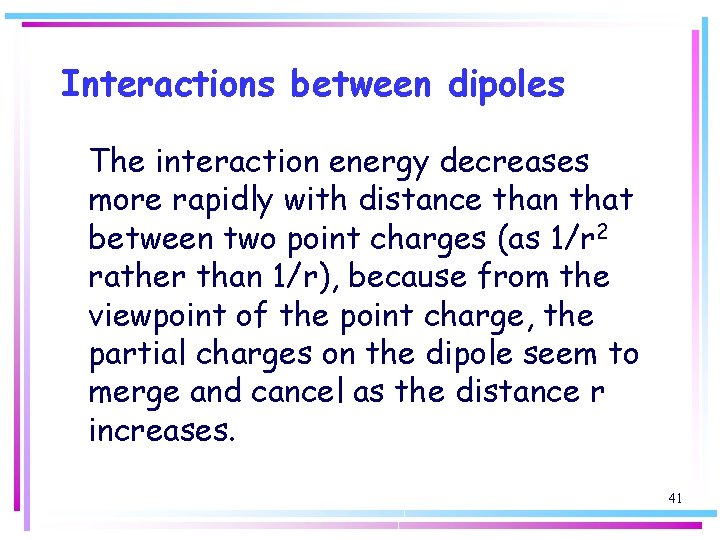
Interactions between dipoles The interaction energy decreases more rapidly with distance than that between two point charges (as 1/r 2 rather than 1/r), because from the viewpoint of the point charge, the partial charges on the dipole seem to merge and cancel as the distance r increases. 41

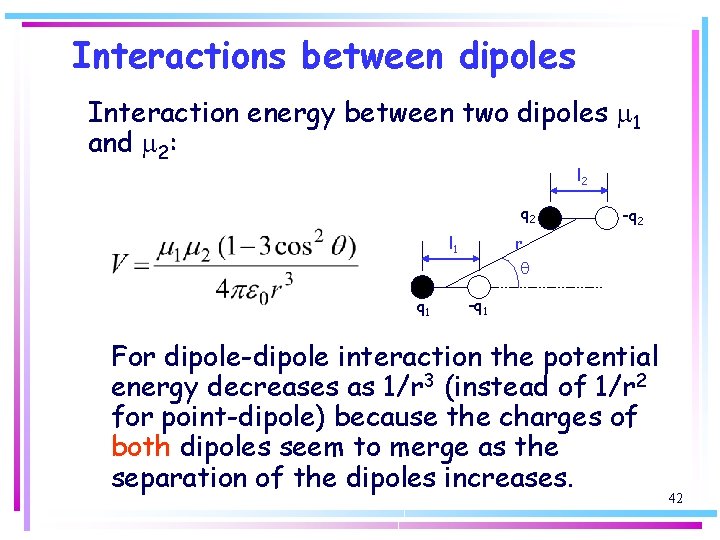
Interactions between dipoles Interaction energy between two dipoles m 1 and m 2: l 2 q 2 l 1 -q 2 r q q 1 -q 1 For dipole-dipole interaction the potential energy decreases as 1/r 3 (instead of 1/r 2 for point-dipole) because the charges of both dipoles seem to merge as the separation of the dipoles increases. 42

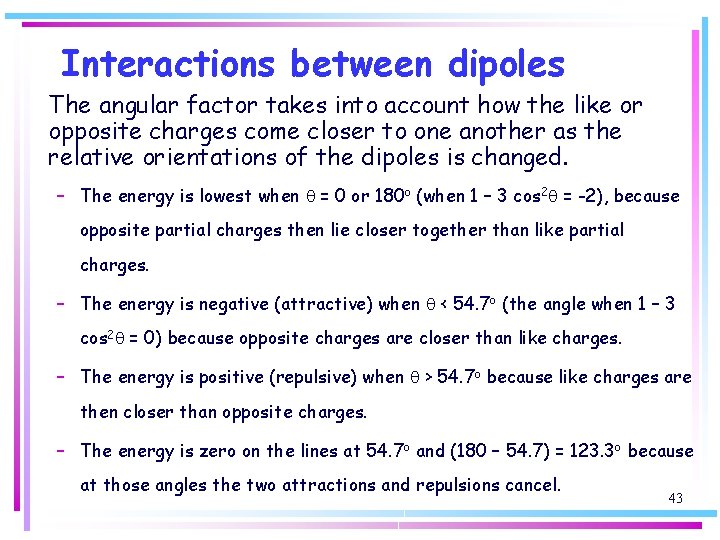
Interactions between dipoles The angular factor takes into account how the like or opposite charges come closer to one another as the relative orientations of the dipoles is changed. – The energy is lowest when q = 0 or 180 o (when 1 – 3 cos 2 q = -2), because opposite partial charges then lie closer together than like partial charges. – The energy is negative (attractive) when q < 54. 7 o (the angle when 1 – 3 cos 2 q = 0) because opposite charges are closer than like charges. – The energy is positive (repulsive) when q > 54. 7 o because like charges are then closer than opposite charges. – The energy is zero on the lines at 54. 7 o and (180 – 54. 7) = 123. 3 o because at those angles the two attractions and repulsions cancel. 43

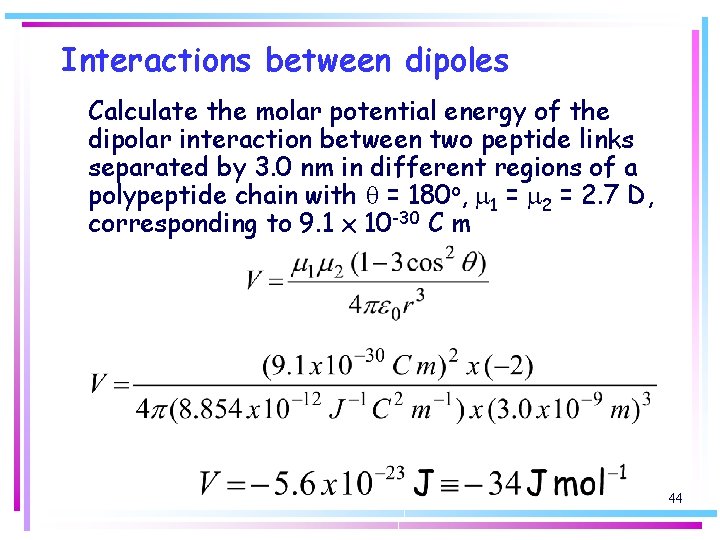
Interactions between dipoles Calculate the molar potential energy of the dipolar interaction between two peptide links separated by 3. 0 nm in different regions of a polypeptide chain with q = 180 o, m 1 = m 2 = 2. 7 D, corresponding to 9. 1 x 10 -30 C m 44

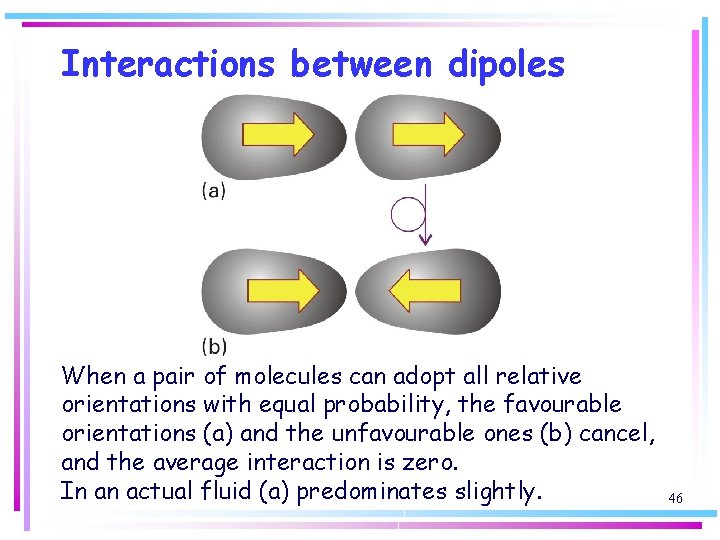
Interactions between dipoles The average energy of interaction between polar molecules that are freely rotating in a fluid (gas or liquid) is zero (attractions and repulsions cancel). However, because the potential energy for dipole-dipole interaction depends on their relative orientations, the molecules exert forces on one another, and do not rotate completely freely, even in a gas. Thus, the lower energy orientations are marginally favoured so there is a non-zero interaction between rotating polar molecules. 45

Interactions between dipoles When a pair of molecules can adopt all relative orientations with equal probability, the favourable orientations (a) and the unfavourable ones (b) cancel, and the average interaction is zero. In an actual fluid (a) predominates slightly. 46

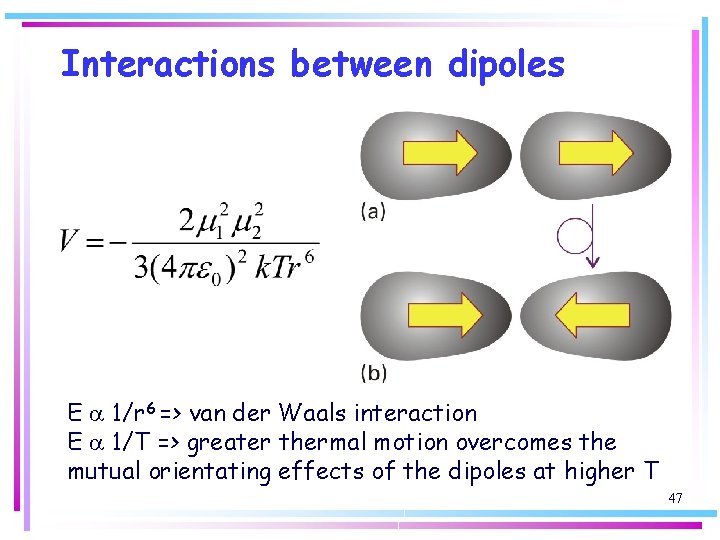
Interactions between dipoles E a 1/r 6 => van der Waals interaction E a 1/T => greater thermal motion overcomes the mutual orientating effects of the dipoles at higher T 47

Interactions between dipoles • At 25 o. C the average interaction energy for pairs of molecules with m = 1 D is about -1. 4 k. J mol-1 when the separation is 0. 3 nm. • This energy is comparable to average molar kinetic energy of 3/2 RT = 3. 7 k. J mol-1 at 25 o. C. • These are similar but much less than the energies involved in the making and breaking of chemical bonds. 48

Induced dipole moments A non-polar molecule may acquire a temporary induced dipole moment m* as a result of the influence of an electric field generated by a nearby ion or polar molecule. The field distorts the electron distribution of the molecule and gives rise to an electric dipole. The molecule is said to be polarizable. The magnitude of the induced dipole moment is proportional to the strength of the electric field, E, giving: m* = a E where a is the polarizability of the molecule. 49

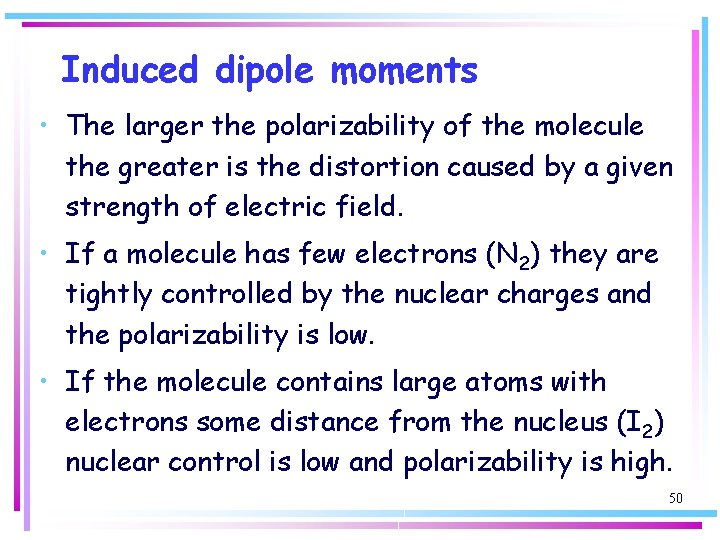
Induced dipole moments • The larger the polarizability of the molecule the greater is the distortion caused by a given strength of electric field. • If a molecule has few electrons (N 2) they are tightly controlled by the nuclear charges and the polarizability is low. • If the molecule contains large atoms with electrons some distance from the nucleus (I 2) nuclear control is low and polarizability is high. 50

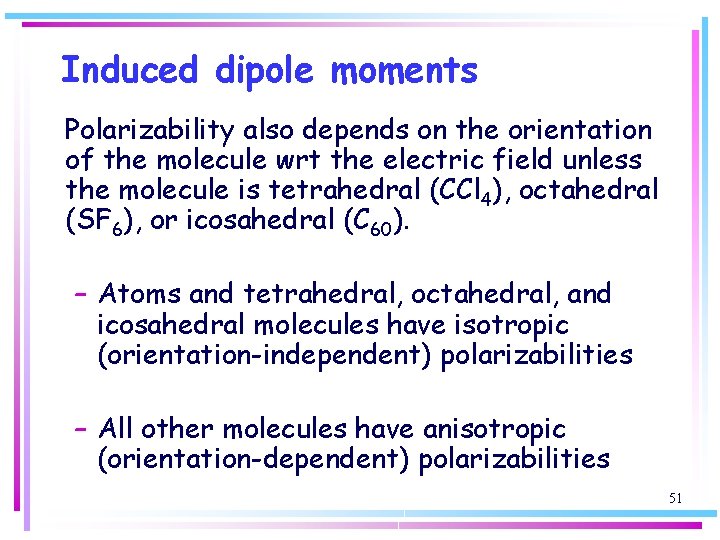
Induced dipole moments Polarizability also depends on the orientation of the molecule wrt the electric field unless the molecule is tetrahedral (CCl 4), octahedral (SF 6), or icosahedral (C 60). – Atoms and tetrahedral, octahedral, and icosahedral molecules have isotropic (orientation-independent) polarizabilities – All other molecules have anisotropic (orientation-dependent) polarizabilities 51

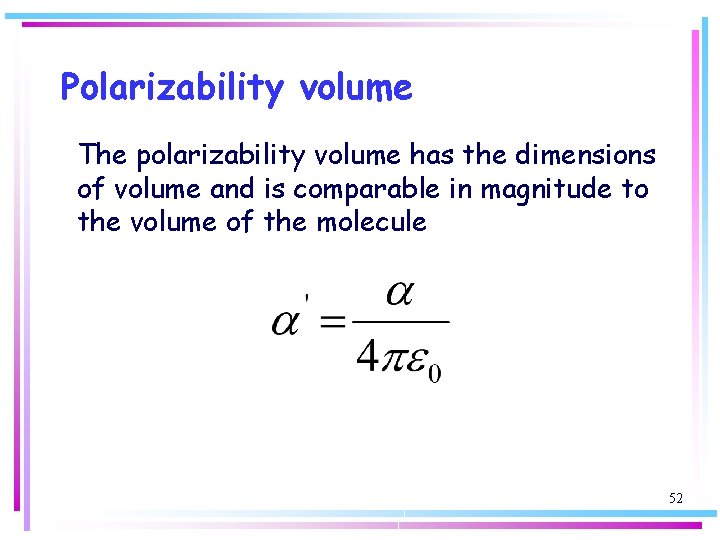
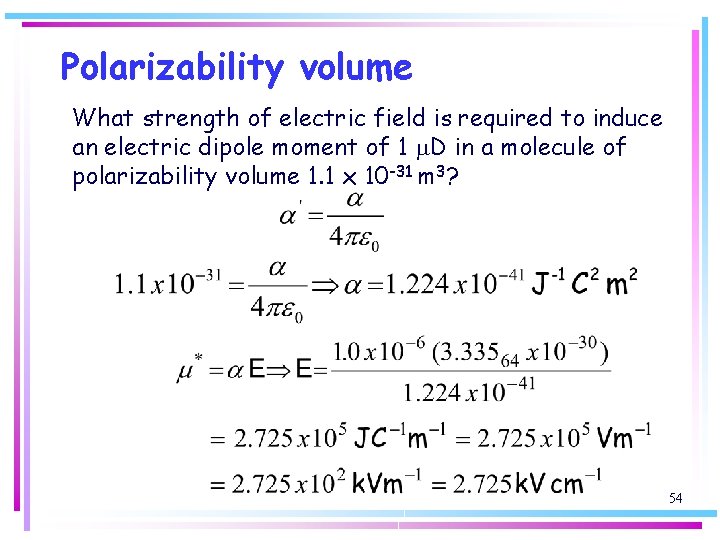
Polarizability volume The polarizability volume has the dimensions of volume and is comparable in magnitude to the volume of the molecule 52

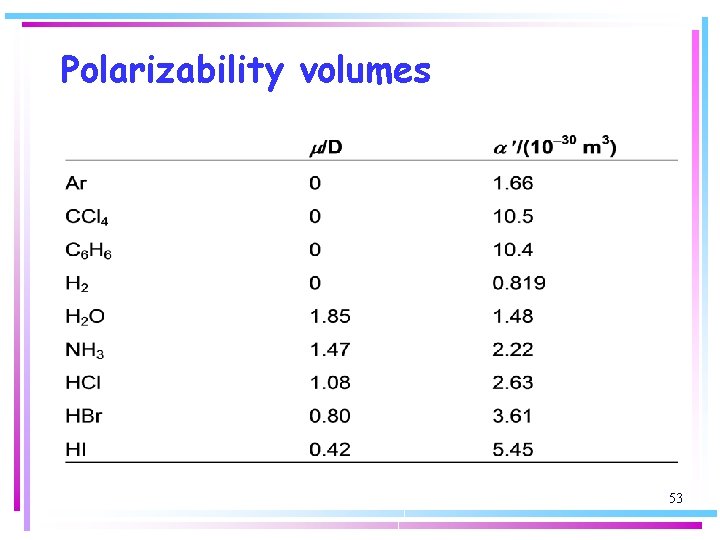
Polarizability volumes 53

Polarizability volume What strength of electric field is required to induce an electric dipole moment of 1 m. D in a molecule of polarizability volume 1. 1 x 10 -31 m 3? 54

Dipole-induced dipole moments A polar molecule with dipole moment m 1 can induce a dipole moment in a polarizable molecule the induced dipole interacts with the permanent dipole of the first molecule and the two are attracted together the induced dipole (light arrows) follows the changing orientation of the permanent dipole (yellow arrows) 55

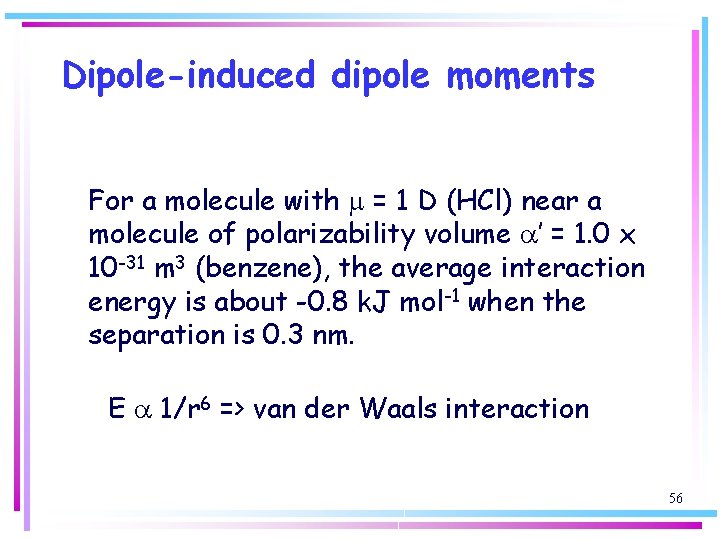
Dipole-induced dipole moments For a molecule with m = 1 D (HCl) near a molecule of polarizability volume a’ = 1. 0 x 10 -31 m 3 (benzene), the average interaction energy is about -0. 8 k. J mol-1 when the separation is 0. 3 nm. E a 1/r 6 => van der Waals interaction 56

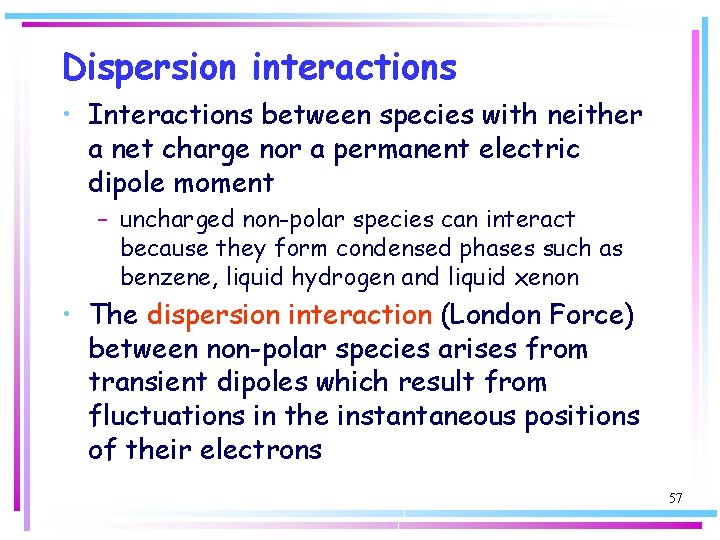
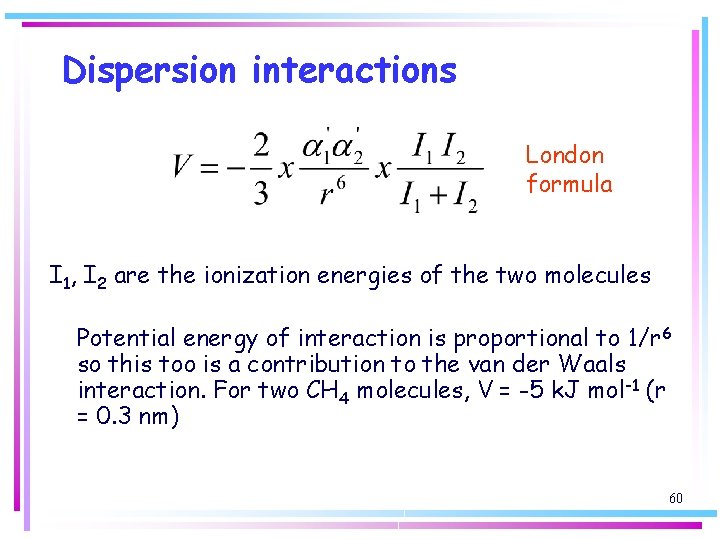
Dispersion interactions • Interactions between species with neither a net charge nor a permanent electric dipole moment – uncharged non-polar species can interact because they form condensed phases such as benzene, liquid hydrogen and liquid xenon • The dispersion interaction (London Force) between non-polar species arises from transient dipoles which result from fluctuations in the instantaneous positions of their electrons 57

Dispersion interactions Electrons from one molecule may flicker into an arrangement that results in partial positive and negative charges and thus gives an instantaneous dipole moment m 1. This dipole can polarize another molecule and induce in it an instantaneous dipole moment m 2. Although the first dipole will go on to change the size and direction of its dipole (≈ 10 -16 s) the second dipole will follow it; the two dipoles are correlated in direction, with the positive charge on one molecule close to a negative partial charge on the other molecule and vice versa. An instantaneous dipole on one molecule induces a dipole on another molecule, and the two dipoles attract thus lowering the energy. 58

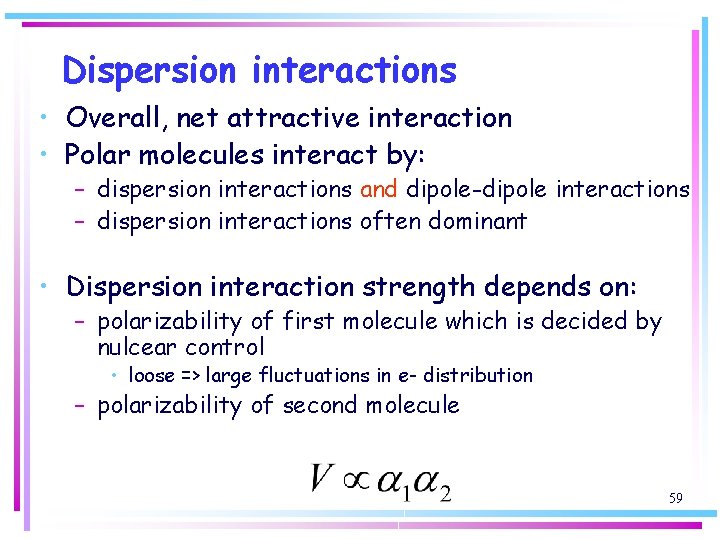
Dispersion interactions • Overall, net attractive interaction • Polar molecules interact by: – dispersion interactions and dipole-dipole interactions – dispersion interactions often dominant • Dispersion interaction strength depends on: – polarizability of first molecule which is decided by nulcear control • loose => large fluctuations in e- distribution – polarizability of second molecule 59

Dispersion interactions London formula I 1, I 2 are the ionization energies of the two molecules Potential energy of interaction is proportional to 1/r 6 so this too is a contribution to the van der Waals interaction. For two CH 4 molecules, V = -5 k. J mol-1 (r = 0. 3 nm) 60

Total interaction- Hydrogen bonding The coulombic interaction between the partly exposed positive charge of a proton bound to an electron withdrawing X atom (in X—H) and the negative charge of a lone pair on the second atom Y, d-X—Hd+ ……Ydas in: • Strongest intermolecular interaction • Denoted X—H……Y, with X and Y being N, O, or F – only molecules with these atoms • ‘Contact’ interaction – turns on when X—H group is in contact with Y atom 61

Hydrogen bonding • Leads to: – – – rigidity of molecular solids (sucrose, ice) low vapour pressure (water) high viscosity (water) high surface tension (water) secondary structure of proteins (helices) attachment of drugs to receptor sites in proteins 62

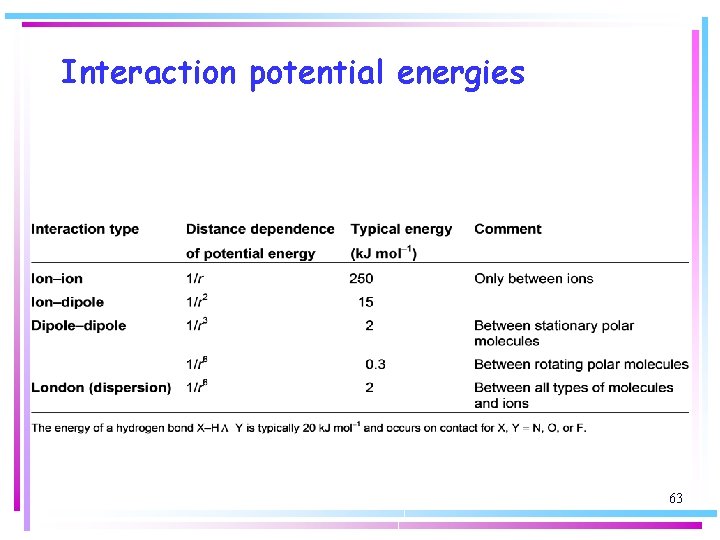
Interaction potential energies 63

The Hydrophobic effect • An apparent force that influences the shape of a macromolecule mediated by the properties of the solvent, water. • Why don’t HC molecules dissolve appreciably in water? • Experiments show that the transfer of a hydrocarbon molecule from a non-polar solvent into water is often exothermic (DH < 0) • The fact that dissolving is not spontaneous must mean that entropy change is negative (DS < 0). 64

The Hydrophobic effect • For example, the process: CH 4 (in CCl 4) = CH 4 (aq) has DH = - 10 k. J mol-1, DS = - 75 J K-1 mol 1, and DG = + 12 k. J mol-1 at 298 K. • Substances characterized by a positive Gibbs energy of transfer from a nonpolar to a polar solvent are classified as hydrophobic. 65

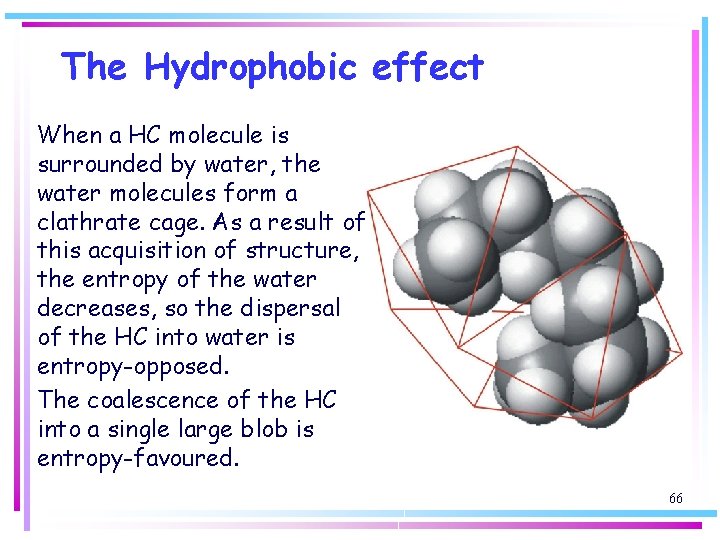
The Hydrophobic effect When a HC molecule is surrounded by water, the water molecules form a clathrate cage. As a result of this acquisition of structure, the entropy of the water decreases, so the dispersal of the HC into water is entropy-opposed. The coalescence of the HC into a single large blob is entropy-favoured. 66

The Hydrophobic effect • The formation of the clathrate cage decreases the entropy of the system because the water molecules must adopt a less disordered arrangement than in the bulk liquid. • However, when many solute molecules cluster together fewer (but larger) cages are required and more solvent molecules are free to move. • This leads to a net decrease in the organization of the solvent and thus a net increase in the entropy of the system. 67

The Hydrophobic effect • This increase in entropy of the solvent is large enough to render spontaneous the association of hydrophobic molecules in a polar solvent. • The increase in entropy that results in the decrease in structural demands on the solvent is the origin of the hydrophobic effect. • The presence of hydrophobic groups in polypeptides results in an increase in structure of the surrounding water molecules and a decrease in entropy. 68

Modelling the total interaction The total attractive interaction energy between rotating molecules that cannot participate in hydrogen bonding is the sum of the contributions from the dipole, dipole-induced-dipole, and dispersion interactions. Only the dispersion interaction contributes if both molecules are nonpolar. 69

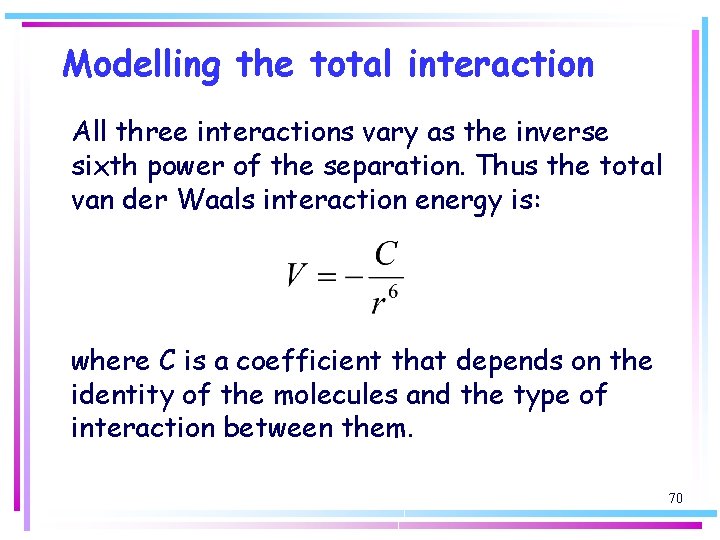
Modelling the total interaction All three interactions vary as the inverse sixth power of the separation. Thus the total van der Waals interaction energy is: where C is a coefficient that depends on the identity of the molecules and the type of interaction between them. 70

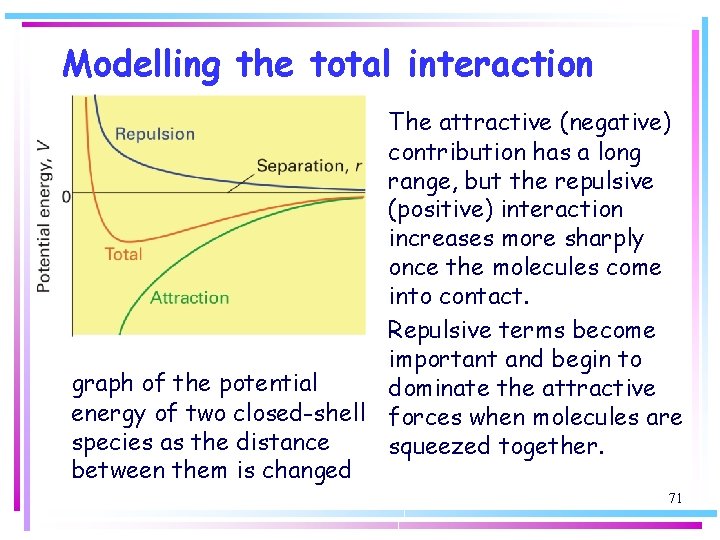
Modelling the total interaction The attractive (negative) contribution has a long range, but the repulsive (positive) interaction increases more sharply once the molecules come into contact. Repulsive terms become important and begin to graph of the potential dominate the attractive energy of two closed-shell forces when molecules are species as the distance squeezed together. between them is changed 71

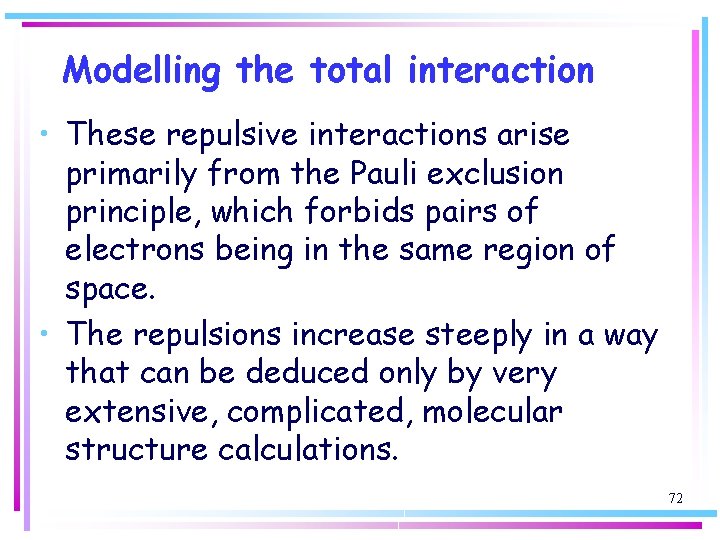
Modelling the total interaction • These repulsive interactions arise primarily from the Pauli exclusion principle, which forbids pairs of electrons being in the same region of space. • The repulsions increase steeply in a way that can be deduced only by very extensive, complicated, molecular structure calculations. 72

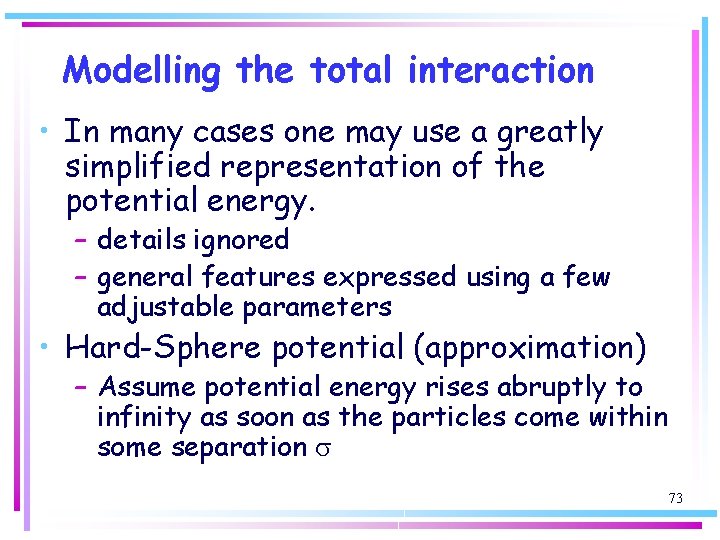
Modelling the total interaction • In many cases one may use a greatly simplified representation of the potential energy. – details ignored – general features expressed using a few adjustable parameters • Hard-Sphere potential (approximation) – Assume potential energy rises abruptly to infinity as soon as the particles come within some separation s 73

Modelling the total interaction • V = ∞ for r ≤ s • V = 0 for r > s s This very simple assumption is surprisingly useful in assessing a number of properties. There is no potential energy of interaction until the two molecules are separated by a distance s when the potential energy rises abruptly to infinity 74

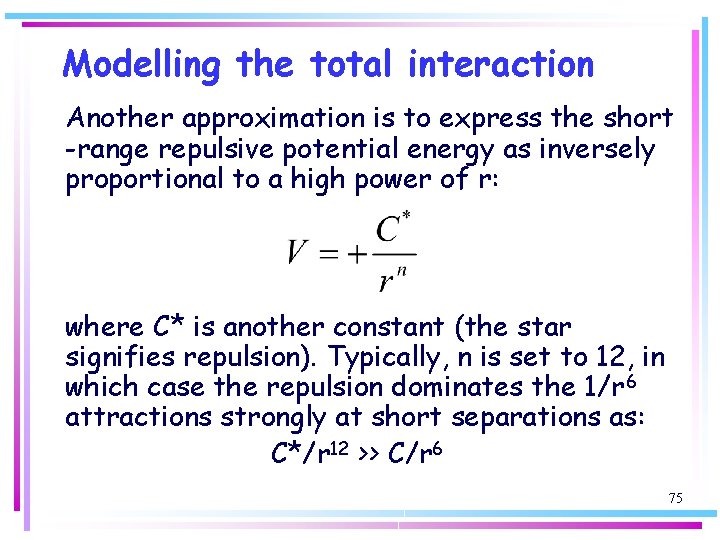
Modelling the total interaction Another approximation is to express the short -range repulsive potential energy as inversely proportional to a high power of r: where C* is another constant (the star signifies repulsion). Typically, n is set to 12, in which case the repulsion dominates the 1/r 6 attractions strongly at short separations as: C*/r 12 >> C/r 6 75

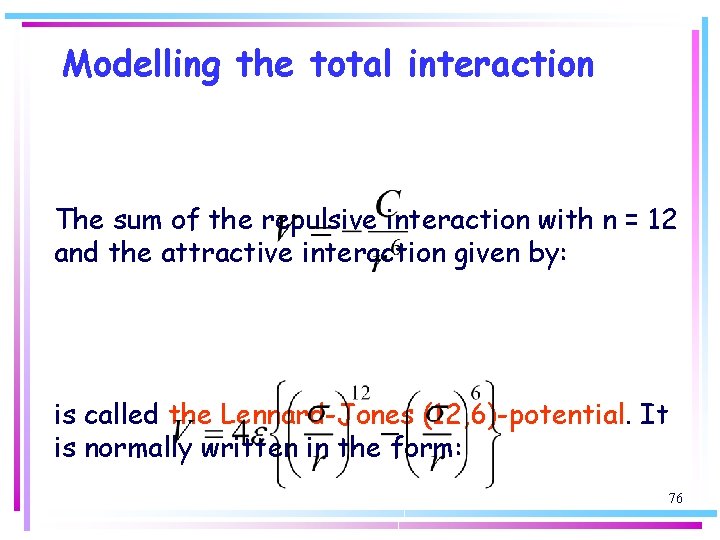
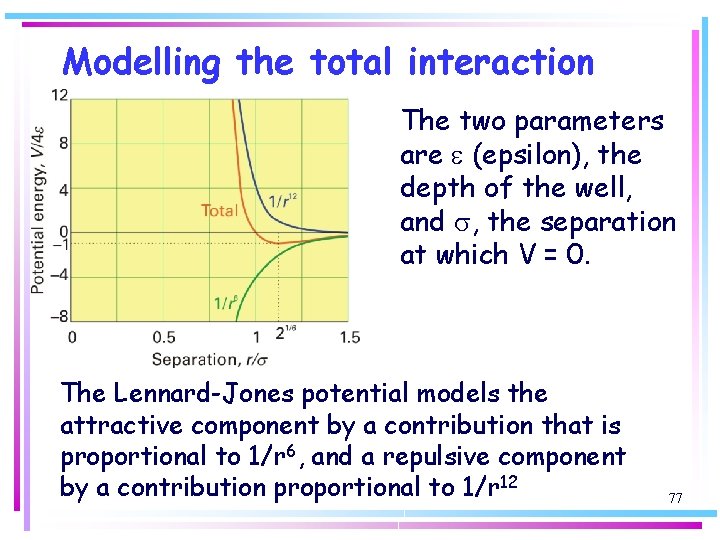
Modelling the total interaction The sum of the repulsive interaction with n = 12 and the attractive interaction given by: is called the Lennard-Jones (12, 6)-potential. It is normally written in the form: 76

Modelling the total interaction The two parameters are e (epsilon), the depth of the well, and s, the separation at which V = 0. The Lennard-Jones potential models the attractive component by a contribution that is proportional to 1/r 6, and a repulsive component by a contribution proportional to 1/r 12 77

Modelling the total interaction Species e/ k. J mol-1 s / pm Ar 128 342 Br 2 536 427 C 6 H 6 454 527 Cl 2 368 412 H 2 34 297 He 11 258 Xe 236 406 78
 Henry curran nuig
Henry curran nuig Meetup galway
Meetup galway Shelley curran
Shelley curran Mary ann curran
Mary ann curran Curran and seaton theory
Curran and seaton theory John curran arin
John curran arin Geoffrey curran
Geoffrey curran John curran arin
John curran arin John curran allen overy
John curran allen overy Bruce curran
Bruce curran Jabbin mulwanda
Jabbin mulwanda Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure đồi và núi
đồi và núi Núi cao bởi có đất bồi
Núi cao bởi có đất bồi Dãy hoàng liên sơn
Dãy hoàng liên sơn Ngày xưa sông núi xinh tươi
Ngày xưa sông núi xinh tươi Châu đại dương tiếp giáp với đại dương nào
Châu đại dương tiếp giáp với đại dương nào Dãy núi cao nhất châu á
Dãy núi cao nhất châu á Dãy núi cao nhất châu á
Dãy núi cao nhất châu á Te pou o te whakaaro nui
Te pou o te whakaaro nui Mặt trời gác núi
Mặt trời gác núi Chúng tôi đứng trên núi chung
Chúng tôi đứng trên núi chung Chiều cao núi phú sĩ
Chiều cao núi phú sĩ Question tags schema
Question tags schema Nui deo
Nui deo Nui natural user interface
Nui natural user interface Bận lòng chi nắm bắt
Bận lòng chi nắm bắt Criptomoedas tcc
Criptomoedas tcc Imagenes de los aymaras
Imagenes de los aymaras ōkole nui
ōkole nui Components of interactive computer graphics are
Components of interactive computer graphics are Nui maynooth library
Nui maynooth library Mặt trời gác núi bóng tối lan dần
Mặt trời gác núi bóng tối lan dần Types of bionicles
Types of bionicles Noncovalent interactions
Noncovalent interactions Interactions among branches of government
Interactions among branches of government Predation symbiotic relationship
Predation symbiotic relationship Interspecific competition definition
Interspecific competition definition Application forms qualitative or quantitative
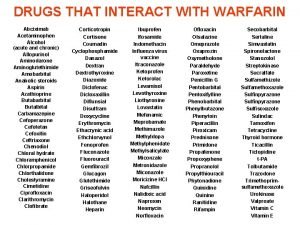
Application forms qualitative or quantitative Sertraline interactions
Sertraline interactions Graphing monetary and fiscal policy interactions
Graphing monetary and fiscal policy interactions The properties and interactions of magnets are called
The properties and interactions of magnets are called Felodapine
Felodapine Interactions in the environment grade 7
Interactions in the environment grade 7 Examples of abiotic factors
Examples of abiotic factors Humans built a dam out of rock materials
Humans built a dam out of rock materials Section 20-1 review species interactions
Section 20-1 review species interactions Plants called sundews have rounded green leaves
Plants called sundews have rounded green leaves Chapter 6 lesson 1 habitats niches and species interactions
Chapter 6 lesson 1 habitats niches and species interactions Ppi omeprazole
Ppi omeprazole Types of interactions
Types of interactions Circulatory system interactions with other systems
Circulatory system interactions with other systems Integral architecture example
Integral architecture example Diaxial interactions
Diaxial interactions Ppi drug interactions
Ppi drug interactions Personal factors in communication
Personal factors in communication Symbiosis and species interactions keystone webquest
Symbiosis and species interactions keystone webquest Functions of the digestive system
Functions of the digestive system Naive bayes pays attention to complex interactions and
Naive bayes pays attention to complex interactions and Chapter 22 reaching out cross-cultural interactions
Chapter 22 reaching out cross-cultural interactions Beri beri
Beri beri Product architectures
Product architectures Special interactions
Special interactions Glipizide interactions
Glipizide interactions Chapter 22 reaching out cross-cultural interactions
Chapter 22 reaching out cross-cultural interactions Chapter 22 reaching out cross-cultural interactions
Chapter 22 reaching out cross-cultural interactions Interactions between ais and internal and external parties
Interactions between ais and internal and external parties Which of the following tells you population density
Which of the following tells you population density Ecosystems interactions
Ecosystems interactions Protein binding interactions
Protein binding interactions Nutrient interactions
Nutrient interactions Foss chemical interactions
Foss chemical interactions Niches and community interactions
Niches and community interactions Interactions
Interactions Synergism
Synergism Chapter 5 evolution and community ecology answer key
Chapter 5 evolution and community ecology answer key Molecular biology
Molecular biology