Molecular Geometry To determine the properties of molecules





- Slides: 12

Molecular Geometry To determine the properties of molecules it is important to know the ______ molecular ______ geometry. To predict molecular shape chemists developed a theory called ________ which VSEPR stands for: Valence Shell Electron Pair _______ Repulsion __________ VSEPR theory says that valence e- repulsion will cause atoms to arrange themselves as far apart as possible. We will study ____ 5 basic molecular geometries with respect to a central (or single) atom. It is necessary to distinguish between ______ bonding pairs of ____ e- and those not shared in a bond. “Unbonded” or elone _______ pair ____ “unpaired” electrons are called _______

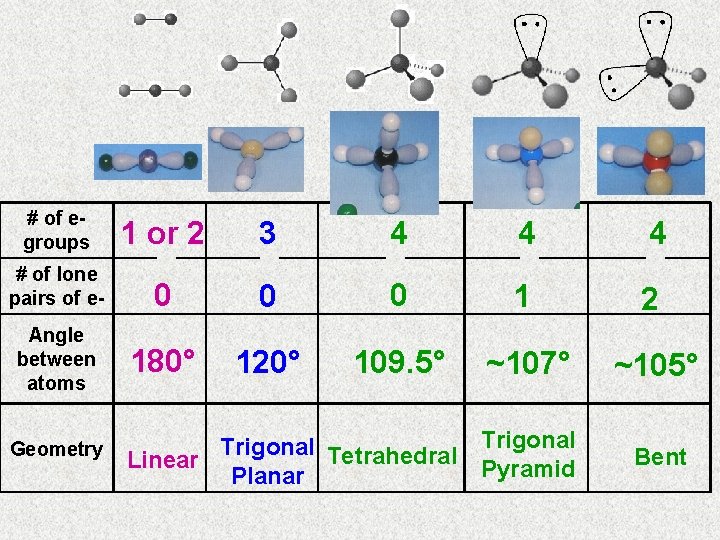
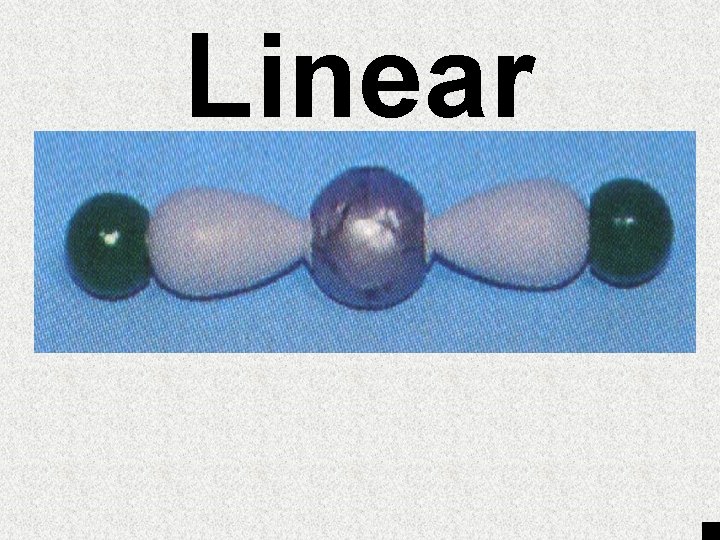
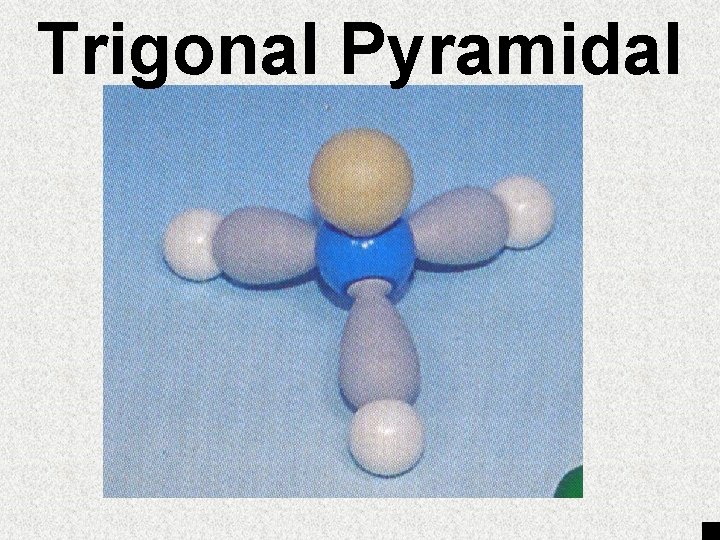
# of egroups 1 or 2 3 4 4 4 # of lone pairs of e- 0 0 0 1 2 Angle between atoms 180° 120° 109. 5° ~107° ~105° Geometry Trigonal Tetrahedral Trigonal Linear Pyramid Planar Bent

Linear

Trigonal Planar

Tetrahedral

Trigonal Pyramidal

Bent

Stability e- These valence shells OR All atoms are surrounded by _____. orbitals full of electrons surrounding each clouds OR _______ repel each other because electron clouds are atom _____ negatively ______ charged and like charges repel. Therefore, atoms molecule arrange themselves as ______ far apart in a _______will minimize the repulsion and as possible from one another to _______ make the molecule stable.

Symmetry Once the molecular geometry of a compound has been symmetry can be determined. A determined, the ________ many or _______ no lines of symmetry. molecule can have ____ Generally, lines of symmetry are found by cutting through the bond between two atoms. _____ Seeing symmetry will take practice!

10 9 8 7 6 5 4 3 2 1 2/6 Molecular Geometry Which of the following molecules has only 1 lone pair of e- on its central atom? Draw each e- dot structure in your notebook and describe the molecular geometry (shape and angle) of each molecule. A) H 2 S (S in the middle) B) CH 3 F (C in the middle) C) PF 3 D) HBr (P in the middle)

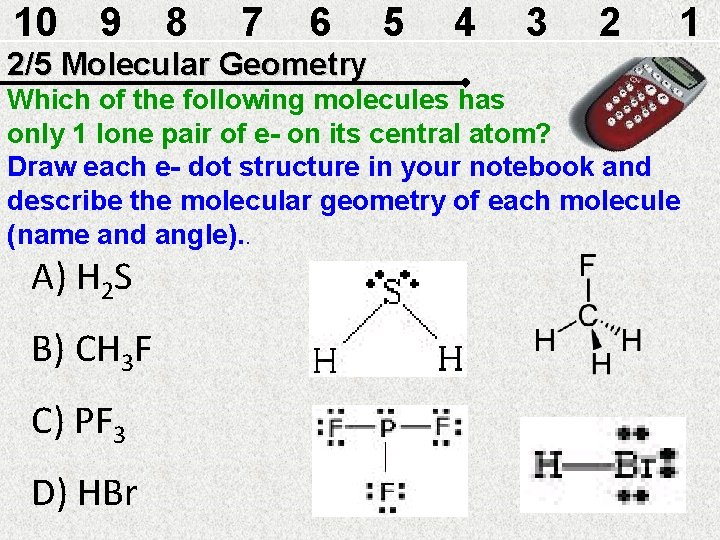
10 9 8 7 6 5 4 3 2 1 2/5 Molecular Geometry Which of the following molecules has only 1 lone pair of e- on its central atom? Draw each e- dot structure in your notebook and describe the molecular geometry of each molecule (name and angle). . A) H 2 S B) CH 3 F C) PF 3 D) HBr

10 9 8 2/6 Symmetry 7 6 5 4 3 2 1 Which of the following molecules is unsymmetrical? In your notebook, draw e- dot structures and label the molecular geometry with bond angles as evidence of your answer. A) Be. Cl 2 B) H 2 CO C) C 2 H 2 D) OF 2