MOLECULAR GEOMETRY MOLECULAR GEOMETRY VSEPR Valence Shell Electron
- Slides: 16

MOLECULAR GEOMETRY

MOLECULAR GEOMETRY VSEPR • Valence Shell Electron Pair Repulsion theory. Molecule adopts the shape that minimizes the electron pair repulsions. • Most important factor in determining geometry is relative repulsion between electron pairs.

VSEPR Theory • Types of e- Pairs – Bonding pairs - form bonds – Lone pairs - nonbonding e- Lone pairs repel more strongly than bonding pairs!!!

VSEPR charts • Use the Lewis structure to determine the geometry of the molecule • How the electrons are arranged determines the bond angles and shapes. • Arrangement focuses on the CENTRAL atom for all data! • Identify REGIONS WHERE ELECTRONS ARE LOCATED (bonds or lone pairs)

Steps to determining geometry 1) Looking at the central atom, how many pairs of electrons surround it? *Double and triple bonds count as ONE pair *Determines “electron pair geometry” 2) How many bonded regions? How many lone pairs? *Determines “molecular geometry”

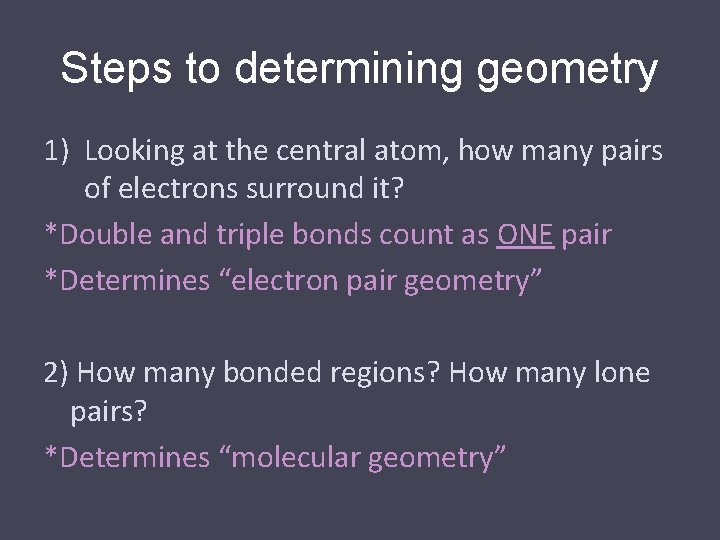
Common Molecular Shapes 2 total 2 bond 0 lone HCN LINEAR 180°

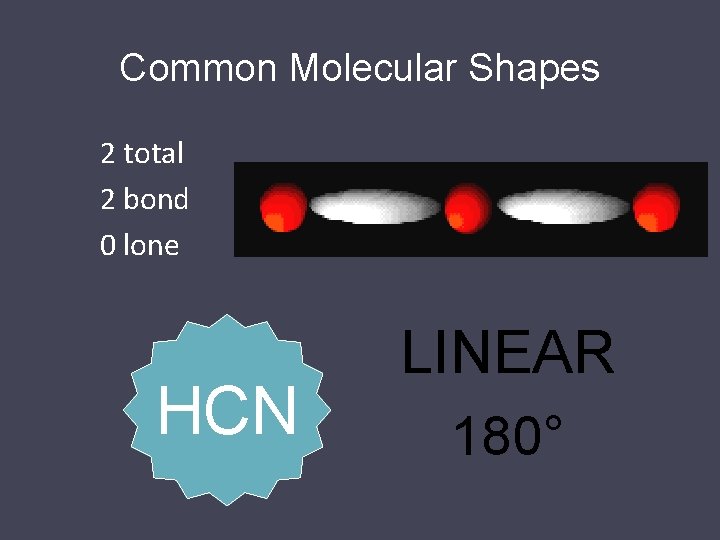
Common Molecular Shapes 3 total 3 bond 0 lone BF 3 TRIGONAL PLANAR 120°

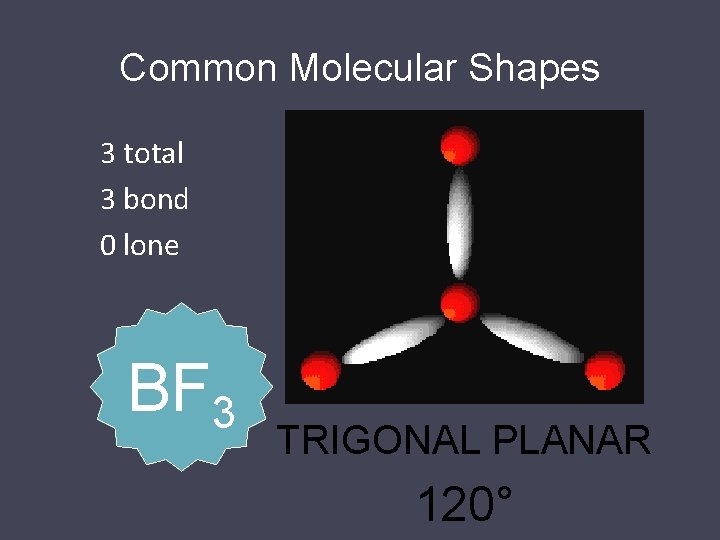
Common Molecular Shapes 3 total 2 bond 1 lone SO 2 BENT 117. 5°

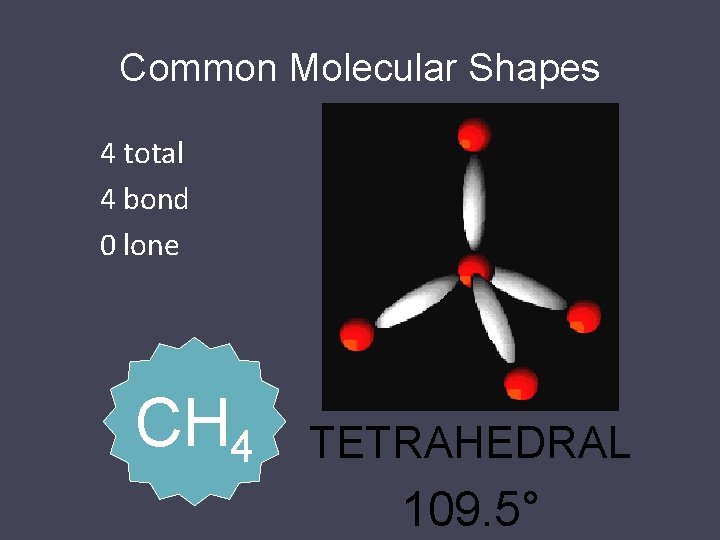
Common Molecular Shapes 4 total 4 bond 0 lone CH 4 TETRAHEDRAL 109. 5°

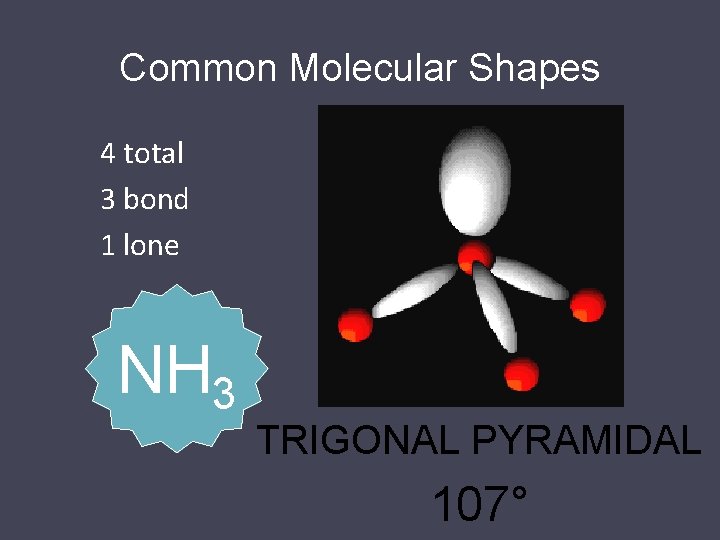
Common Molecular Shapes 4 total 3 bond 1 lone NH 3 TRIGONAL PYRAMIDAL 107°

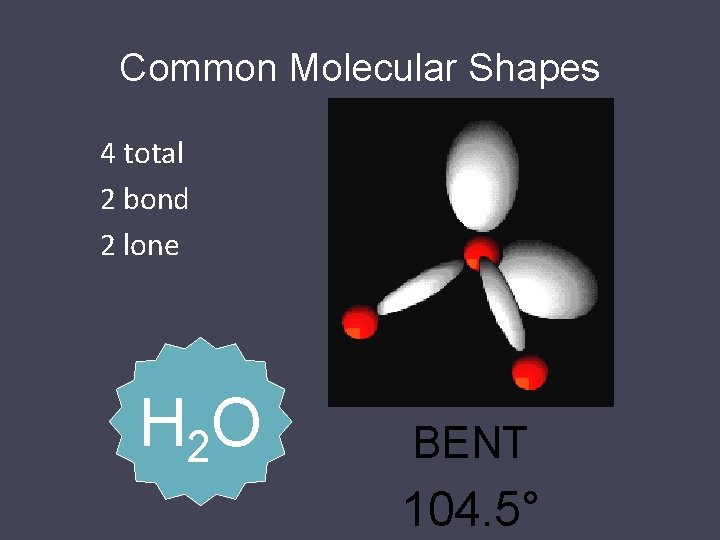
Common Molecular Shapes 4 total 2 bond 2 lone H 2 O BENT 104. 5°

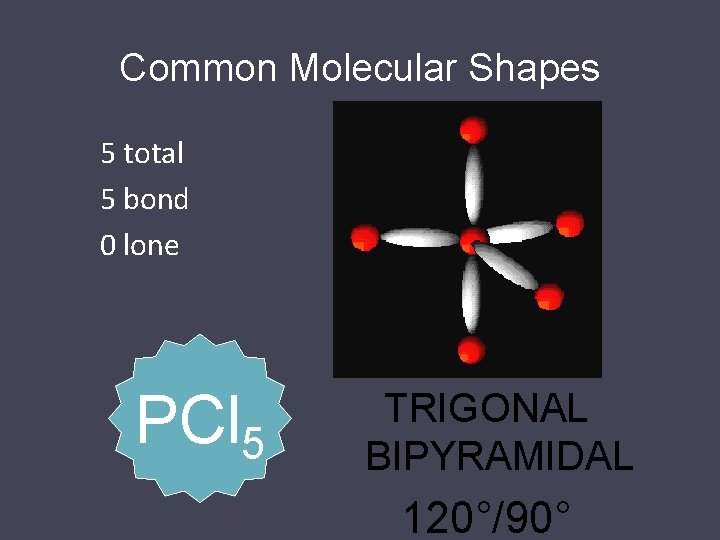
Common Molecular Shapes 5 total 5 bond 0 lone PCl 5 TRIGONAL BIPYRAMIDAL 120°/90°

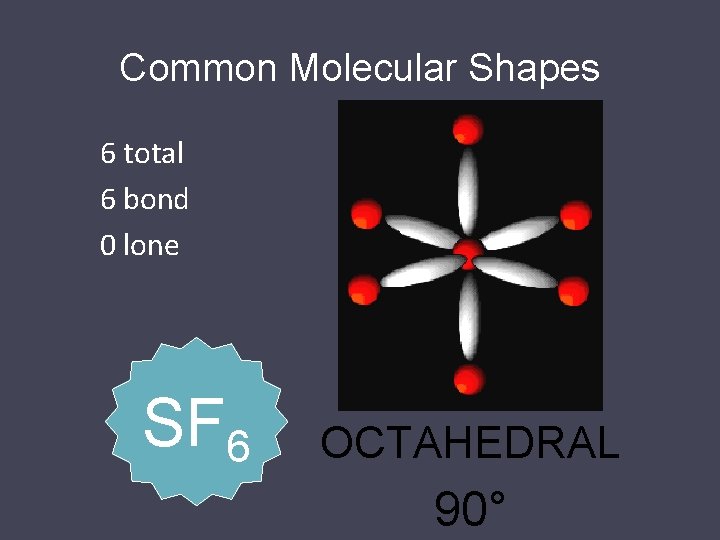
Common Molecular Shapes 6 total 6 bond 0 lone SF 6 OCTAHEDRAL 90°

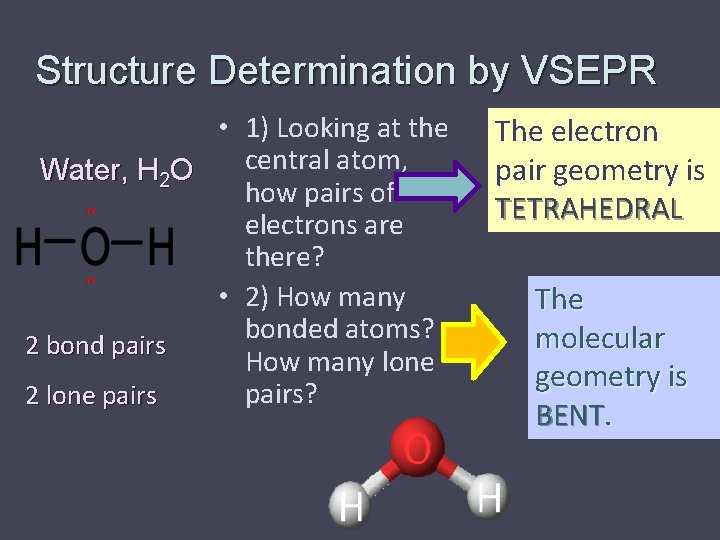
Structure Determination by VSEPR • 1) Looking at the central atom, Water, H 2 O how pairs of electrons are there? • 2) How many bonded atoms? 2 bond pairs How many lone pairs? 2 lone pairs The electron pair geometry is TETRAHEDRAL The molecular geometry is BENT.

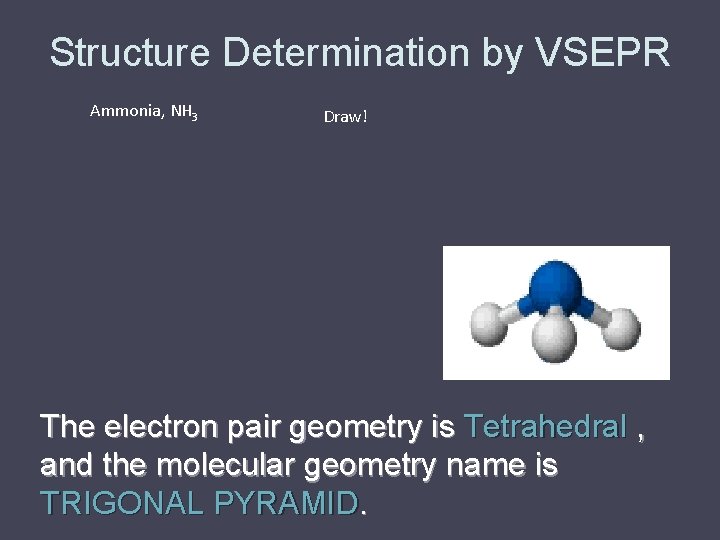
Structure Determination by VSEPR Ammonia, NH 3 Draw! The electron pair geometry is Tetrahedral , and the molecular geometry name is TRIGONAL PYRAMID.

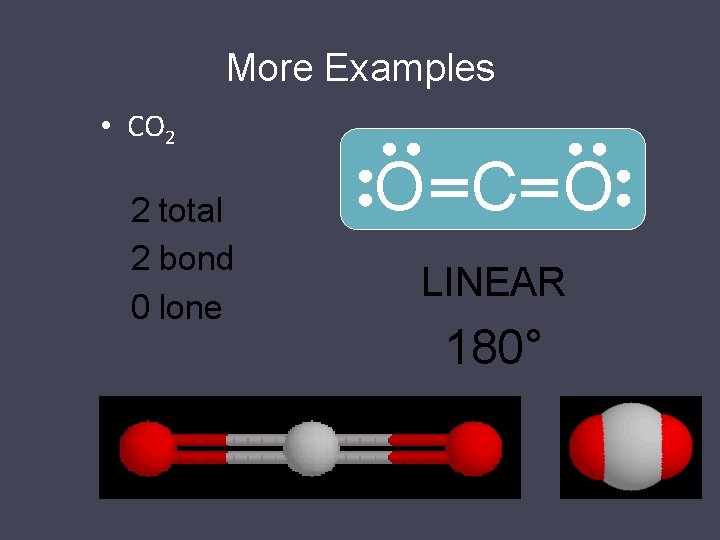
More Examples • CO 2 2 total 2 bond 0 lone O C O LINEAR 180°