Molecular Geometry I VSEPR Theory Electrostatic repulsion causes



















- Slides: 31

Molecular Geometry

I. VSEPR Theory: Electrostatic repulsion causes valence electron pairs to be oriented as far apart as possible.

VSEPR Valence Shell Electron Pair Repulsion

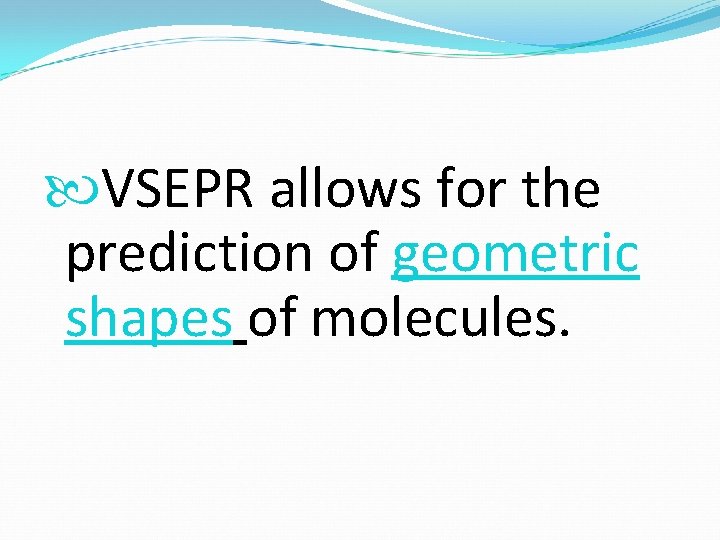
VSEPR allows for the prediction of geometric shapes of molecules.

Geometric shape is based on atoms and unshared pairs of electrons that are attached to the central atom.

Unshared pairs are NOT the same as bonded pairs; unshared pairs push a little harder!

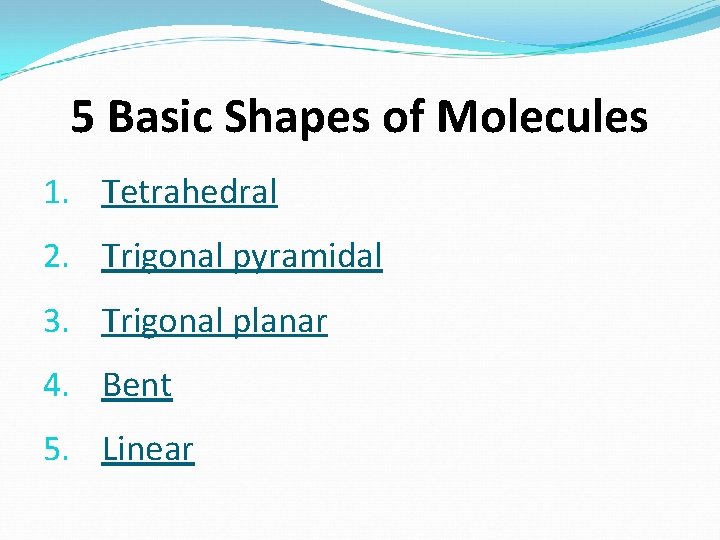
5 Basic Shapes of Molecules 1. Tetrahedral 2. Trigonal pyramidal 3. Trigonal planar 4. Bent 5. Linear

and take the rest of today’s notes

II. Hybridization

• Valence electrons are found in s orbitals and p orbitals • You would expect s to s bonds would be different than s to p bonds or p to p bonds, but they are not. All bonds are identical.

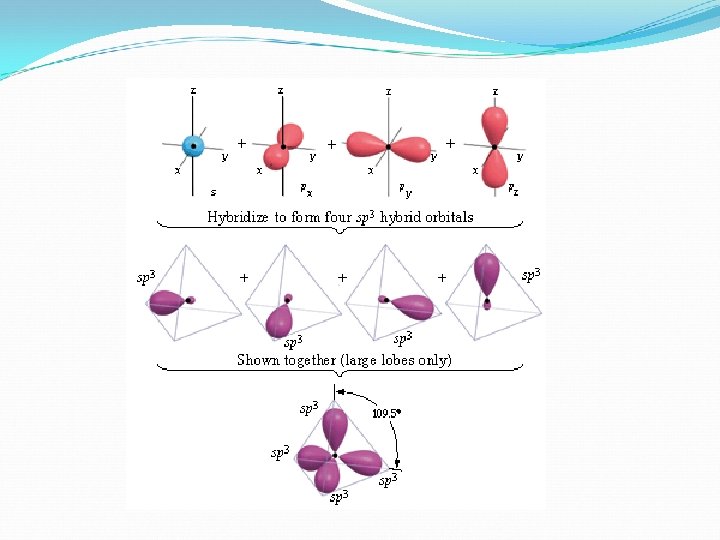
Hybridization- the combining of two or more orbitals of similar energies on the same atom to give new orbitals of equal energy.

Orbitals blend to form special orbitals for bonding. Blended orbitals are hybridized.

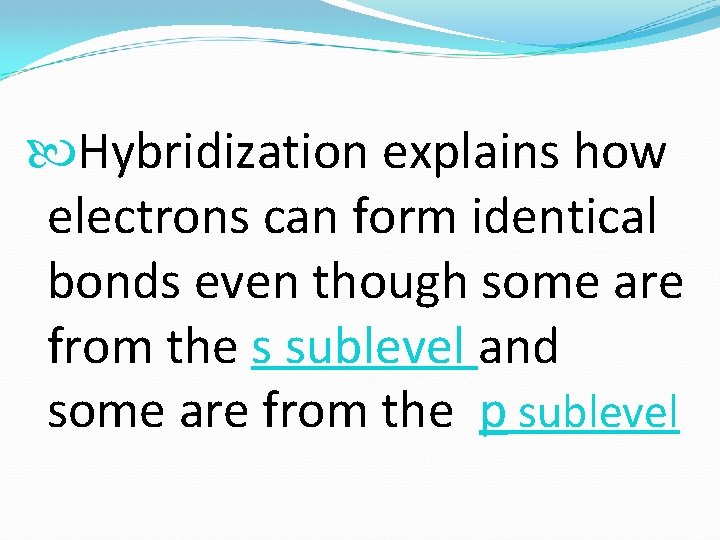
Hybridization explains how electrons can form identical bonds even though some are from the s sublevel and some are from the p sublevel

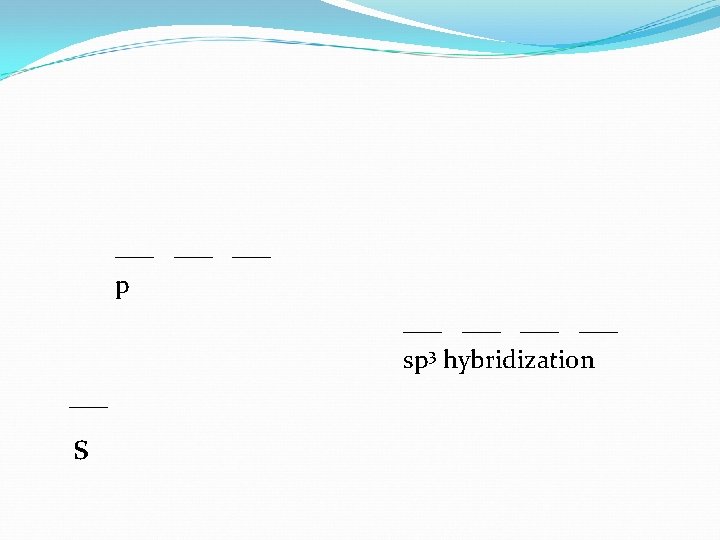
___ ___ p ___ ___ sp 3 hybridization ___ s

Sigma and Pi Bonds Single covalent bonds are also called sigma bonds. Sigma bonds are formed when orbitals blend between the two sharing atoms (hybrid orbitals. )

Pi bonds are the additional bonds that form from overlapping p orbitals. They only form after a sigma bond is formed.

single bond – 1 sigma double bond – 1 sigma, 1 pi triple bond – 1 sigma, 2 pi

How to Determine Hybrid Orbitals The number of hybrid orbitals is determined by the number of sigma bonds and unshared electron pairs on the central atom.

Hybrid notation indicates which orbitals are occupied.


And take the rest of the hybridization notes

III. Polarity

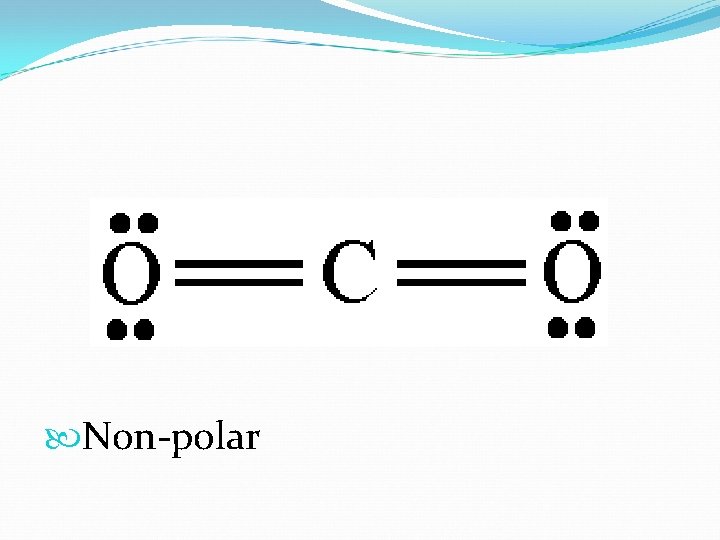
Molecular Polarity A polar molecule has + and – areas within it. Molecules can be even if their bonds are polar: CO 2

So, how do you know? Determine the distribution of electrons within the molecule.

If uneven (not identical) distribution, then molecule is POLAR.

If even distribution (identical), then molecule is NON-POLAR.

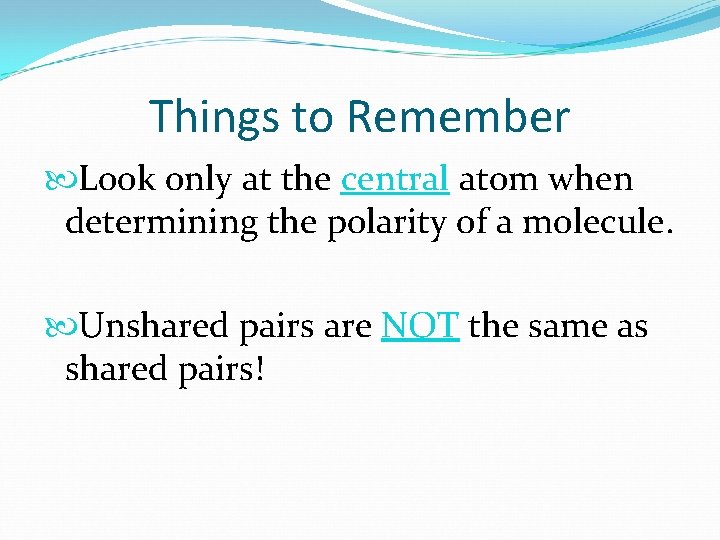
Things to Remember Look only at the central atom when determining the polarity of a molecule. Unshared pairs are NOT the same as shared pairs!

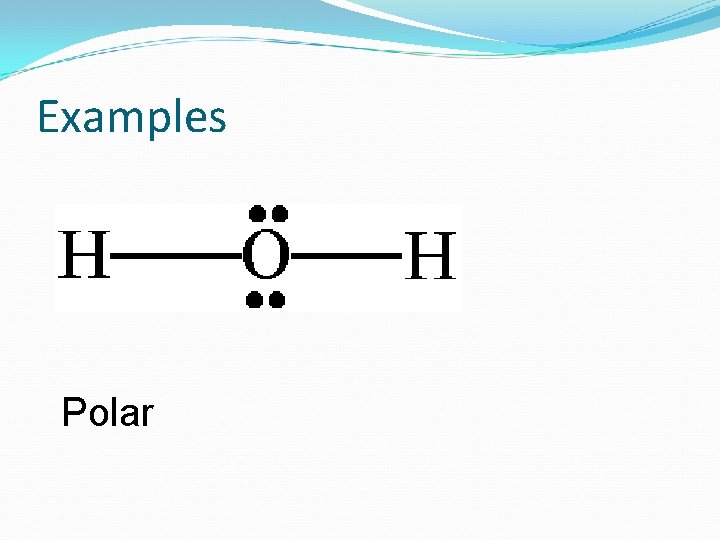
Examples Polar

Non-polar

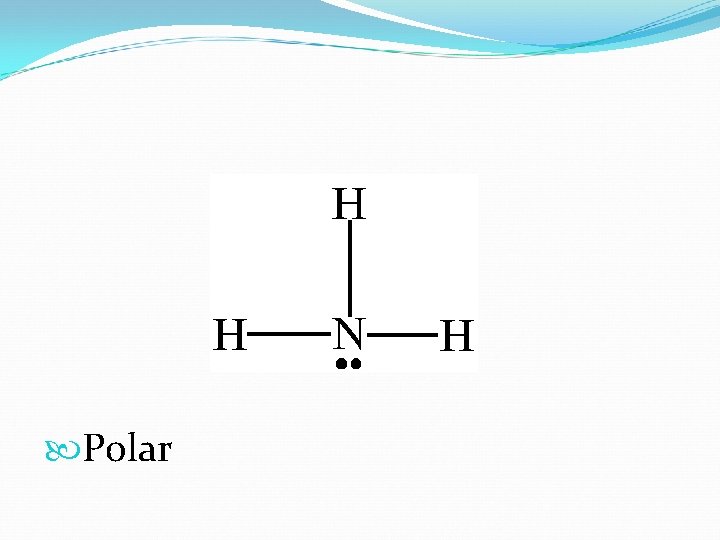
Polar

And do Polarity homework