Molecular Geometry I Lewis Structures A Localized Electron
- Slides: 9

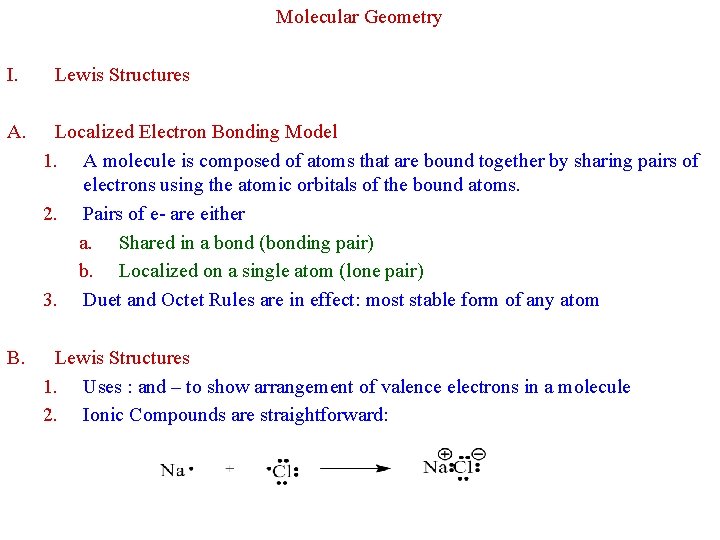
Molecular Geometry I. Lewis Structures A. Localized Electron Bonding Model 1. A molecule is composed of atoms that are bound together by sharing pairs of electrons using the atomic orbitals of the bound atoms. 2. Pairs of e- are either a. Shared in a bond (bonding pair) b. Localized on a single atom (lone pair) 3. Duet and Octet Rules are in effect: most stable form of any atom B. Lewis Structures 1. Uses : and – to show arrangement of valence electrons in a molecule 2. Ionic Compounds are straightforward:

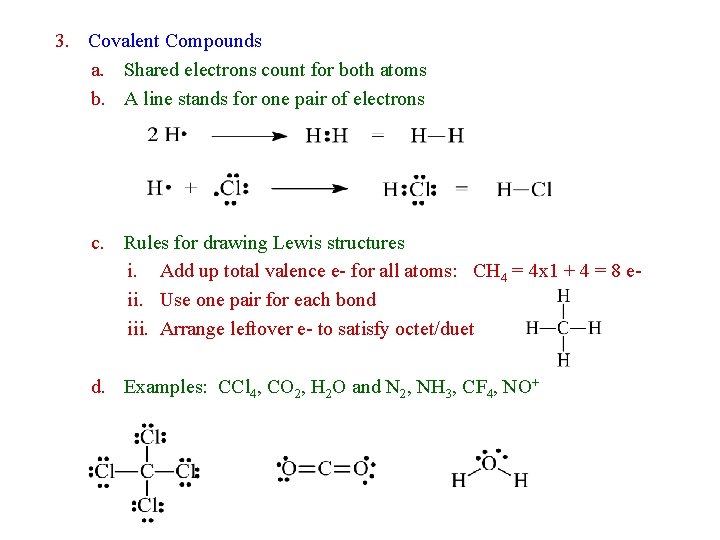
3. Covalent Compounds a. Shared electrons count for both atoms b. A line stands for one pair of electrons c. Rules for drawing Lewis structures i. Add up total valence e- for all atoms: CH 4 = 4 x 1 + 4 = 8 eii. Use one pair for each bond iii. Arrange leftover e- to satisfy octet/duet d. Examples: CCl 4, CO 2, H 2 O and N 2, NH 3, CF 4, NO+

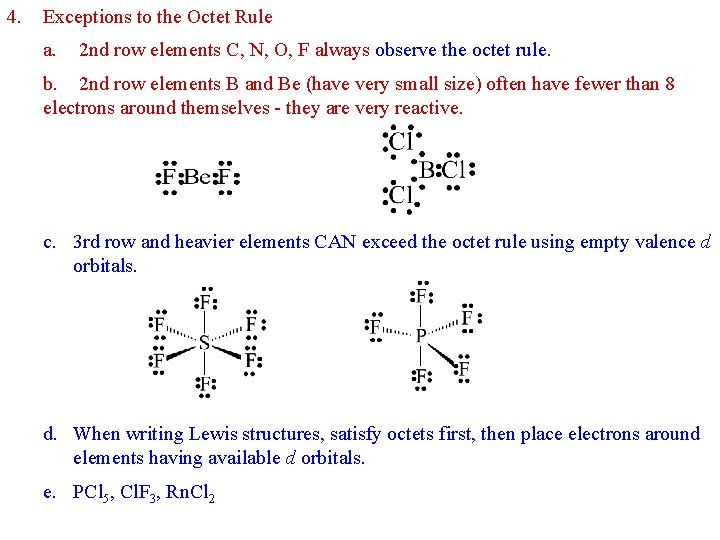
4. Exceptions to the Octet Rule a. 2 nd row elements C, N, O, F always observe the octet rule. b. 2 nd row elements B and Be (have very small size) often have fewer than 8 electrons around themselves - they are very reactive. c. 3 rd row and heavier elements CAN exceed the octet rule using empty valence d orbitals. d. When writing Lewis structures, satisfy octets first, then place electrons around elements having available d orbitals. e. PCl 5, Cl. F 3, Rn. Cl 2

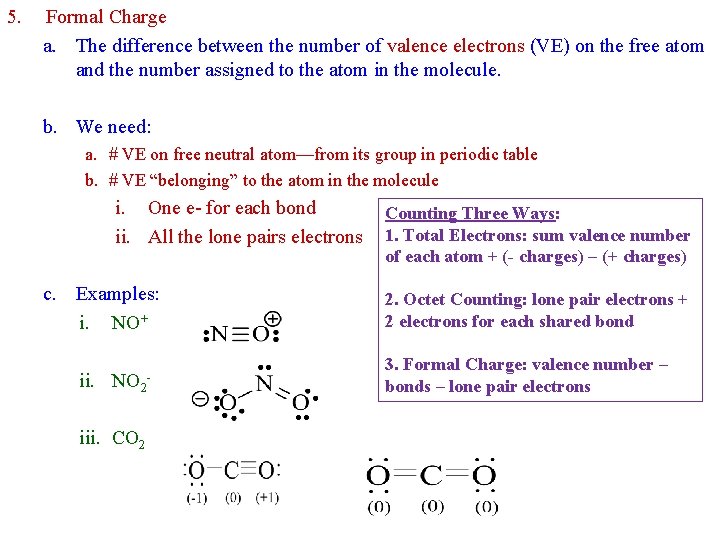
5. Formal Charge a. The difference between the number of valence electrons (VE) on the free atom and the number assigned to the atom in the molecule. b. We need: a. # VE on free neutral atom—from its group in periodic table b. # VE “belonging” to the atom in the molecule i. One e- for each bond ii. All the lone pairs electrons c. Examples: i. NO+ ii. NO 2 iii. CO 2 - Counting Three Ways: 1. Total Electrons: sum valence number of each atom + (- charges) – (+ charges) 2. Octet Counting: lone pair electrons + 2 electrons for each shared bond 3. Formal Charge: valence number – bonds – lone pair electrons

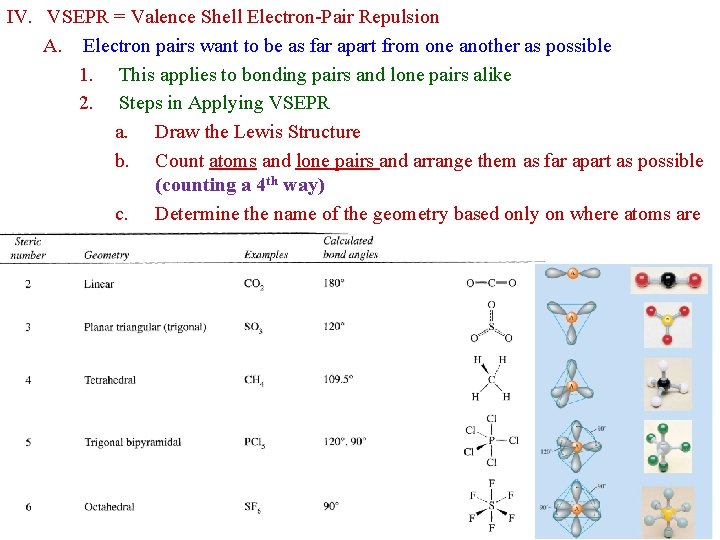
IV. VSEPR = Valence Shell Electron-Pair Repulsion A. Electron pairs want to be as far apart from one another as possible 1. This applies to bonding pairs and lone pairs alike 2. Steps in Applying VSEPR a. Draw the Lewis Structure b. Count atoms and lone pairs and arrange them as far apart as possible (counting a 4 th way) c. Determine the name of the geometry based only on where atoms are

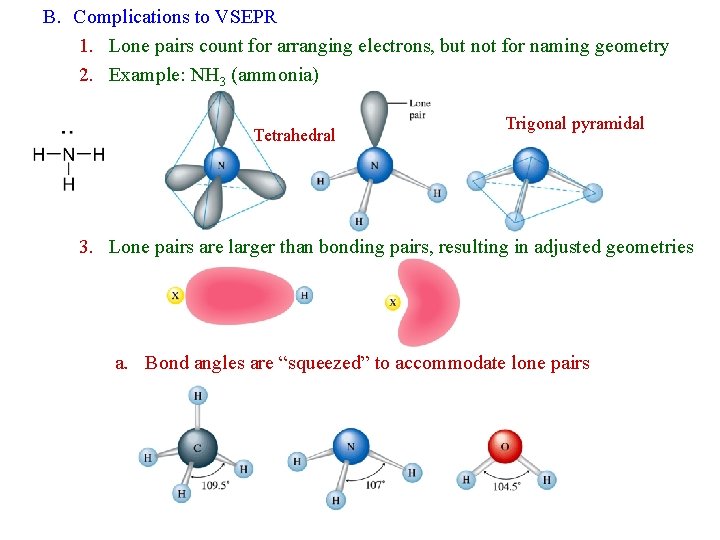
B. Complications to VSEPR 1. Lone pairs count for arranging electrons, but not for naming geometry 2. Example: NH 3 (ammonia) Tetrahedral Trigonal pyramidal 3. Lone pairs are larger than bonding pairs, resulting in adjusted geometries a. Bond angles are “squeezed” to accommodate lone pairs

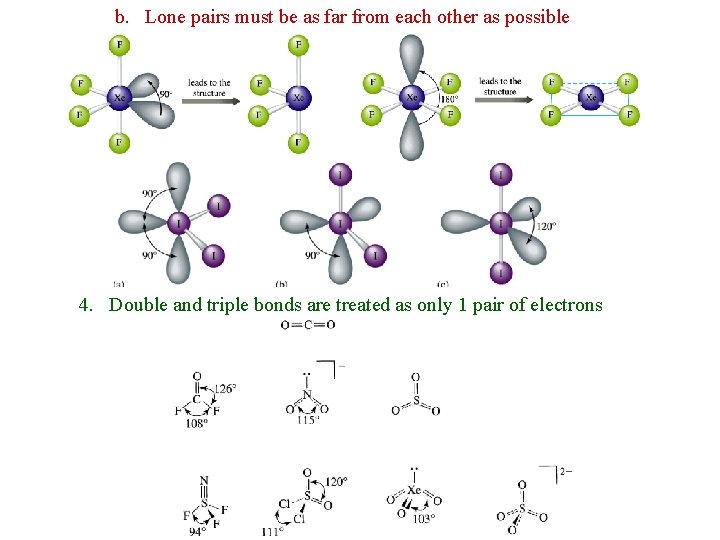
b. Lone pairs must be as far from each other as possible 4. Double and triple bonds are treated as only 1 pair of electrons

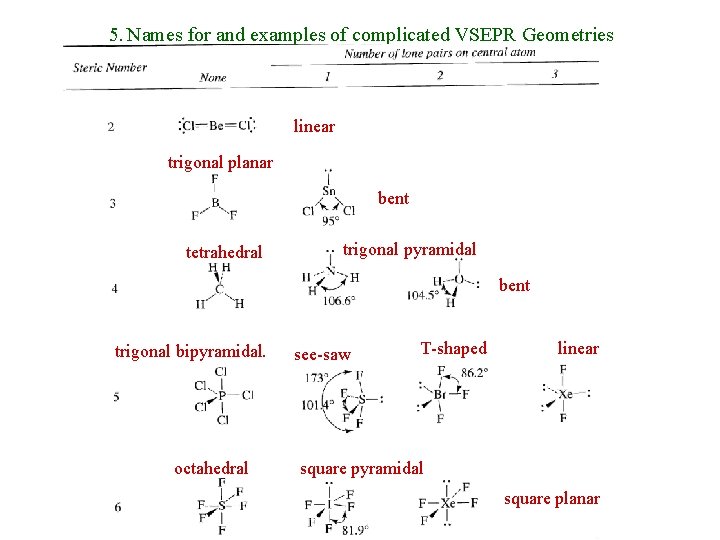
5. Names for and examples of complicated VSEPR Geometries linear trigonal planar bent tetrahedral trigonal pyramidal bent trigonal bipyramidal. octahedral see-saw T-shaped linear square pyramidal square planar

Incident: General Lab Cleanliness and Order