MOLECULAR GEOMETRY Determining the Structure of Molecules Molecular

- Slides: 40

MOLECULAR GEOMETRY Determining the Structure of Molecules

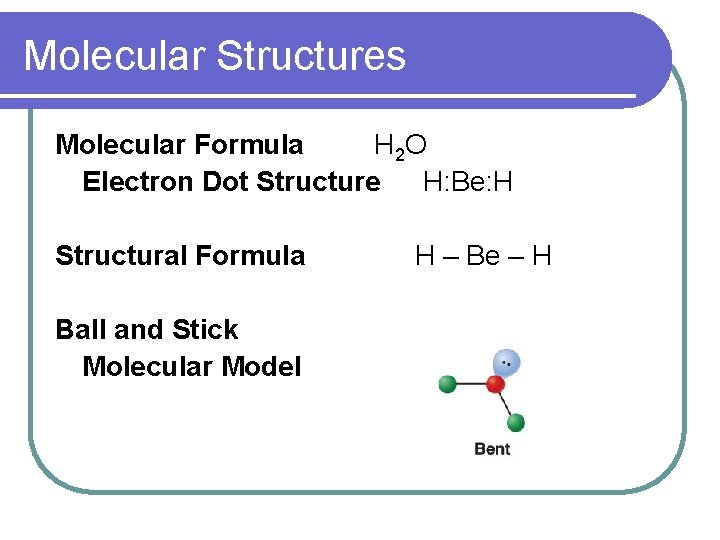
Molecular Structures Molecular Formula H 2 O Electron Dot Structure H: Be: H Structural Formula Ball and Stick Molecular Model H – Be – H

MOLECULAR GEOMETRY Structural formulas, such as NH 3, provide information about bonding only. It does not provide direct information about the shape of the bond or the shape of the molecule. l The repulsion between charge clouds in the outer levels of atoms determines the arrangement of the orbitals. The orbital arrangement determines the shape of the molecules. l

VSEPR l l l Valence Shell Electron Pair Repulsion theory is based on the number of regions of high electron density around a central atom. can be used to predict structures of molecules or ions by minimizing the electrostatic repulsion between the regions of high electron density. can also be used to predict structures of molecules or ions that contain multiple bonds or unpaired electrons. does fail in some cases.

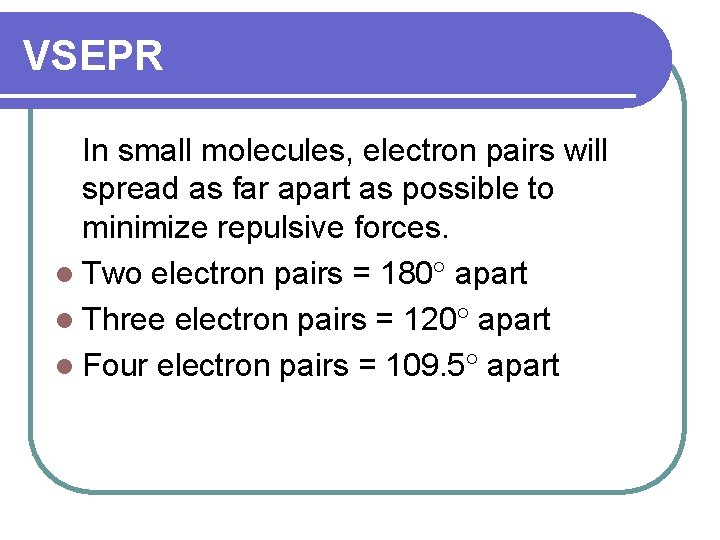
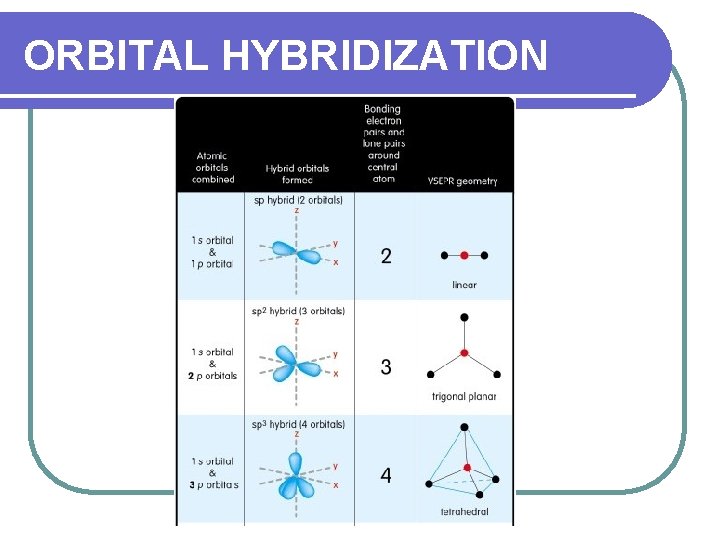
VSEPR In small molecules, electron pairs will spread as far apart as possible to minimize repulsive forces. l Two electron pairs = 180 apart l Three electron pairs = 120 apart l Four electron pairs = 109. 5 apart

SHAPES WE WILL LEARN l Linear l Trigonal Planar l Tetrahedral l Pyramidal l Bent

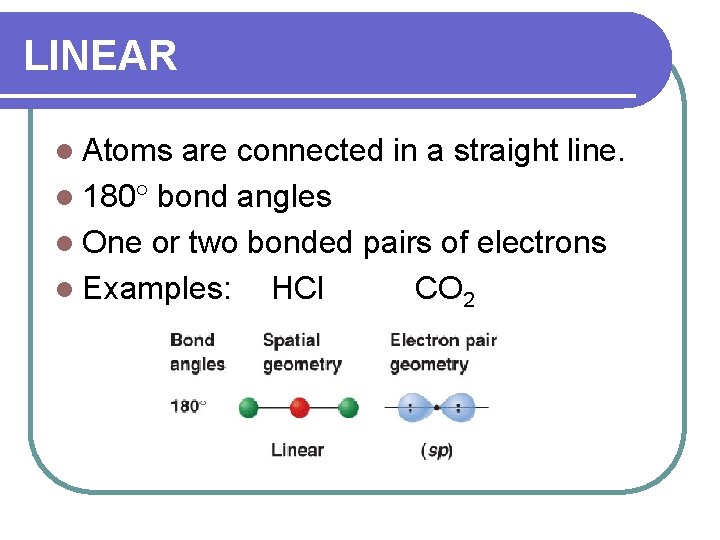
LINEAR l Atoms are connected in a straight line. l 180 bond angles l One or two bonded pairs of electrons l Examples: HCl CO 2

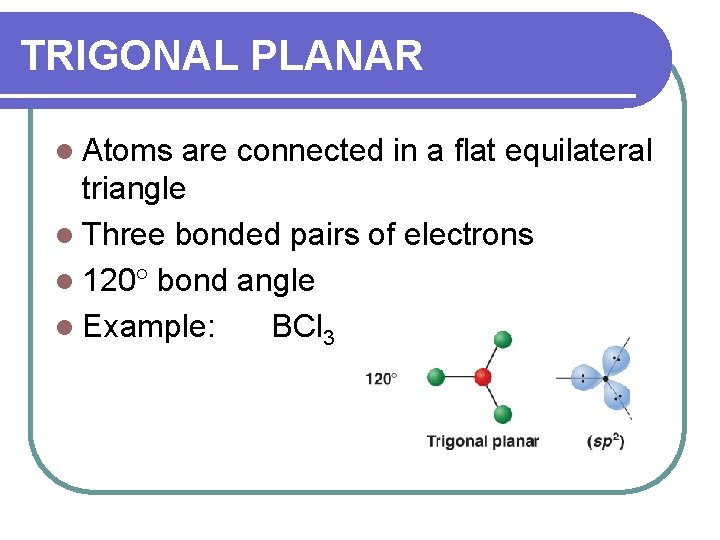
TRIGONAL PLANAR l Atoms are connected in a flat equilateral triangle l Three bonded pairs of electrons l 120 bond angle l Example: BCl 3

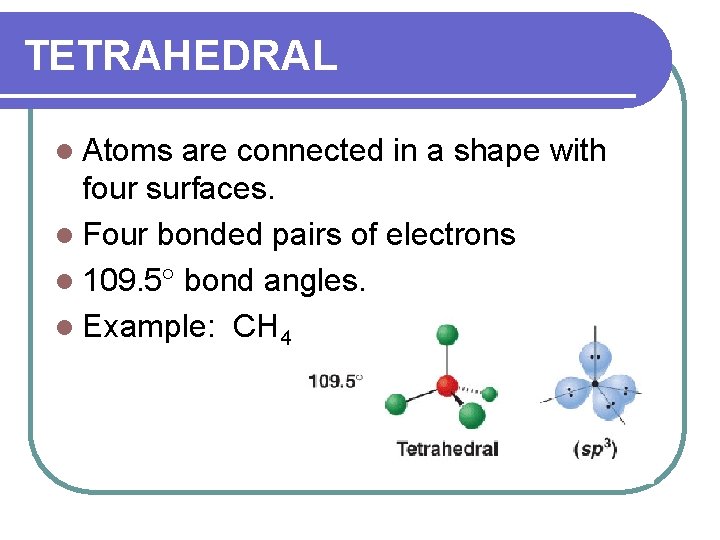
TETRAHEDRAL l Atoms are connected in a shape with four surfaces. l Four bonded pairs of electrons l 109. 5 bond angles. l Example: CH 4

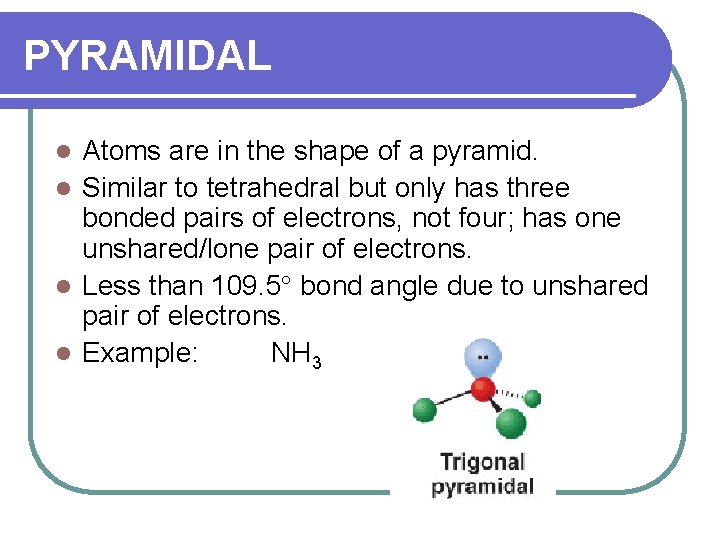
PYRAMIDAL Atoms are in the shape of a pyramid. l Similar to tetrahedral but only has three bonded pairs of electrons, not four; has one unshared/lone pair of electrons. l Less than 109. 5 bond angle due to unshared pair of electrons. l Example: NH 3 l

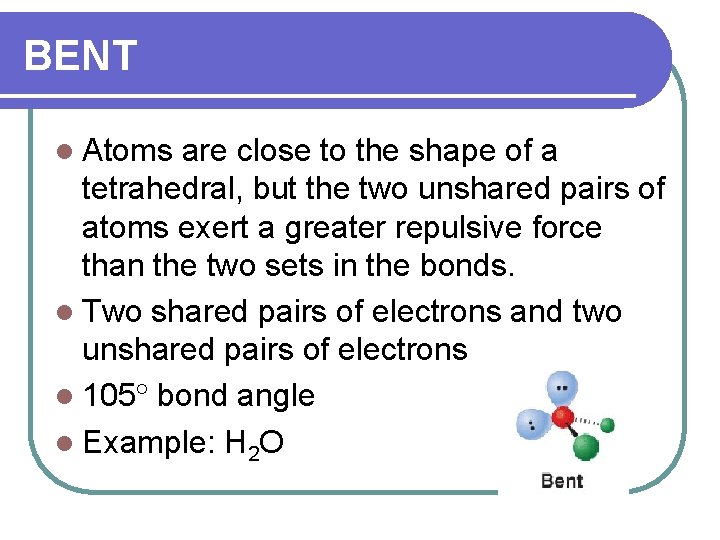
BENT l Atoms are close to the shape of a tetrahedral, but the two unshared pairs of atoms exert a greater repulsive force than the two sets in the bonds. l Two shared pairs of electrons and two unshared pairs of electrons l 105 bond angle l Example: H 2 O

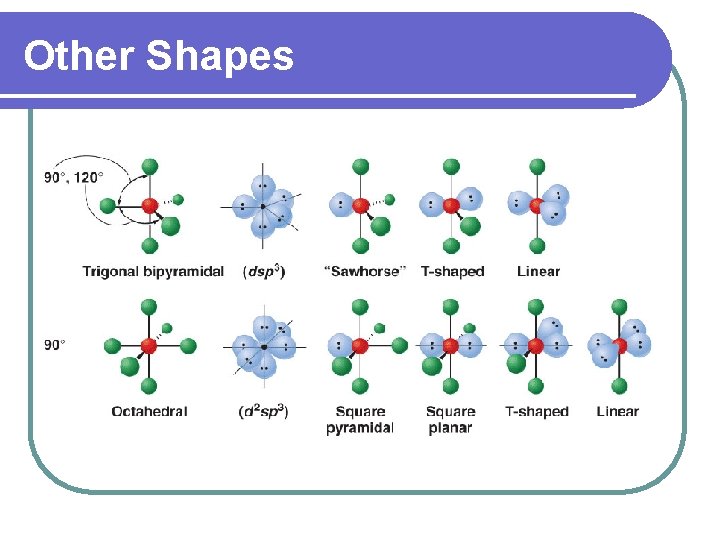
Other Shapes

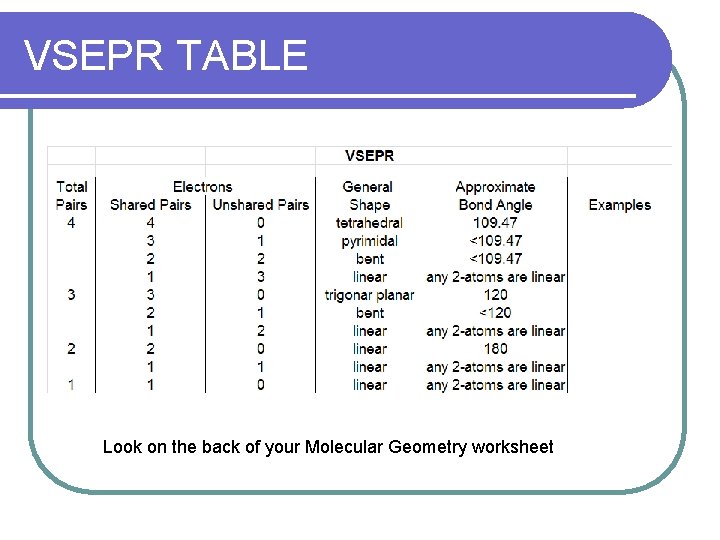
VSEPR TABLE Look on the back of your Molecular Geometry worksheet

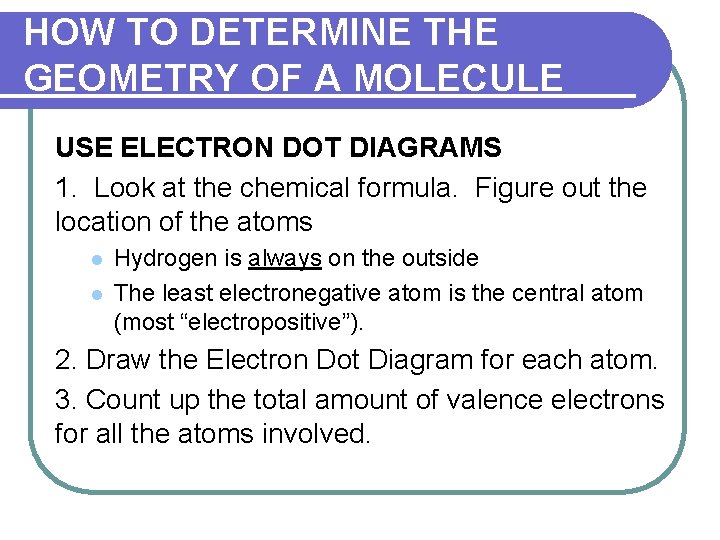
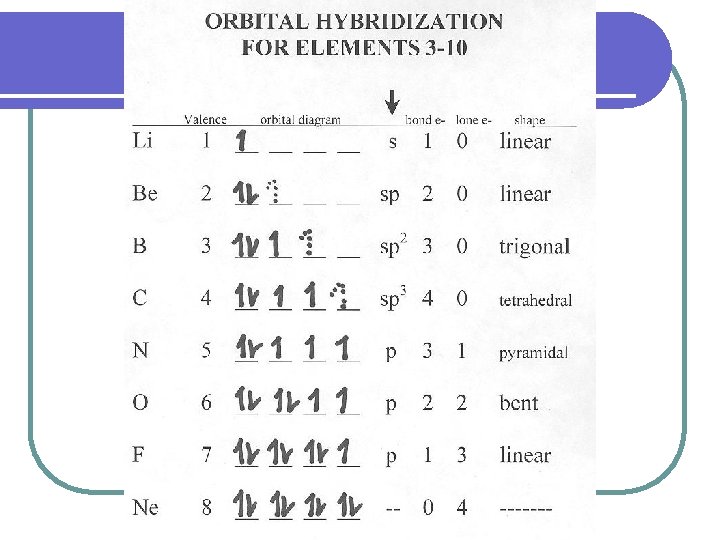
HOW TO DETERMINE THE GEOMETRY OF A MOLECULE USE ELECTRON DOT DIAGRAMS 1. Look at the chemical formula. Figure out the location of the atoms l l Hydrogen is always on the outside The least electronegative atom is the central atom (most “electropositive”). 2. Draw the Electron Dot Diagram for each atom. 3. Count up the total amount of valence electrons for all the atoms involved.

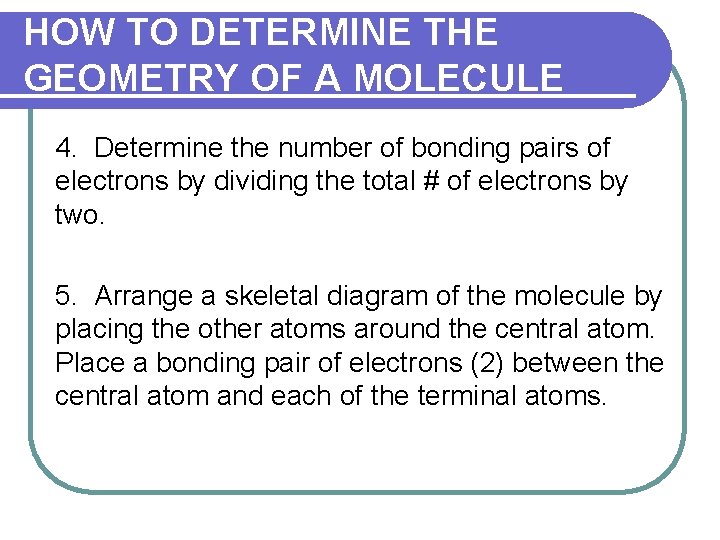
HOW TO DETERMINE THE GEOMETRY OF A MOLECULE 4. Determine the number of bonding pairs of electrons by dividing the total # of electrons by two. 5. Arrange a skeletal diagram of the molecule by placing the other atoms around the central atom. Place a bonding pair of electrons (2) between the central atom and each of the terminal atoms.

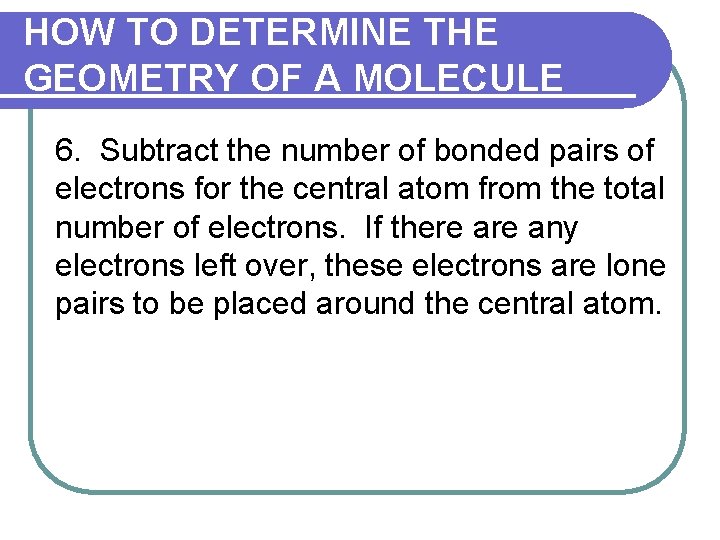
HOW TO DETERMINE THE GEOMETRY OF A MOLECULE 6. Subtract the number of bonded pairs of electrons for the central atom from the total number of electrons. If there any electrons left over, these electrons are lone pairs to be placed around the central atom.

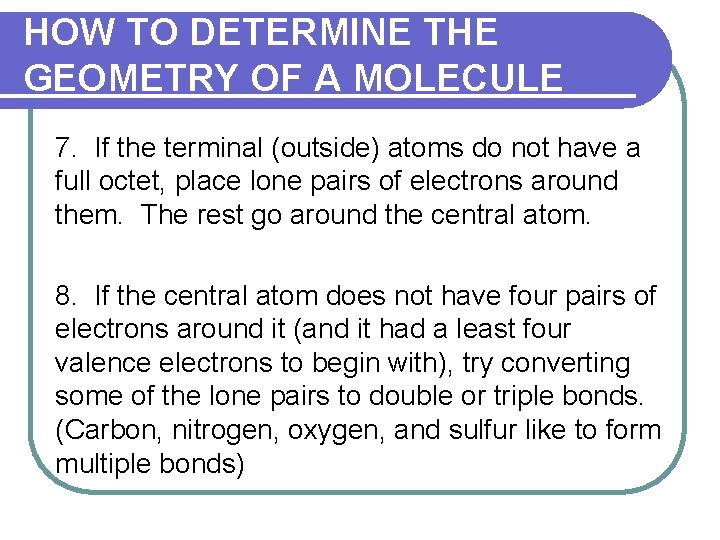
HOW TO DETERMINE THE GEOMETRY OF A MOLECULE 7. If the terminal (outside) atoms do not have a full octet, place lone pairs of electrons around them. The rest go around the central atom. 8. If the central atom does not have four pairs of electrons around it (and it had a least four valence electrons to begin with), try converting some of the lone pairs to double or triple bonds. (Carbon, nitrogen, oxygen, and sulfur like to form multiple bonds)

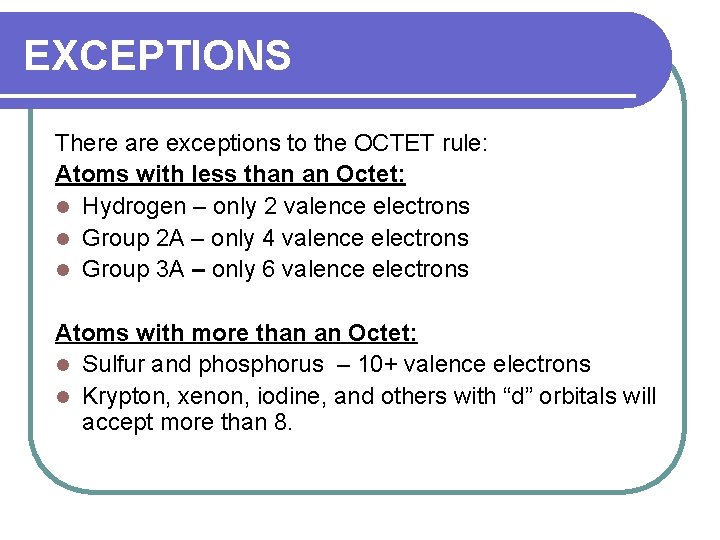
EXCEPTIONS There are exceptions to the OCTET rule: Atoms with less than an Octet: l Hydrogen – only 2 valence electrons l Group 2 A – only 4 valence electrons l Group 3 A – only 6 valence electrons Atoms with more than an Octet: l Sulfur and phosphorus – 10+ valence electrons l Krypton, xenon, iodine, and others with “d” orbitals will accept more than 8.

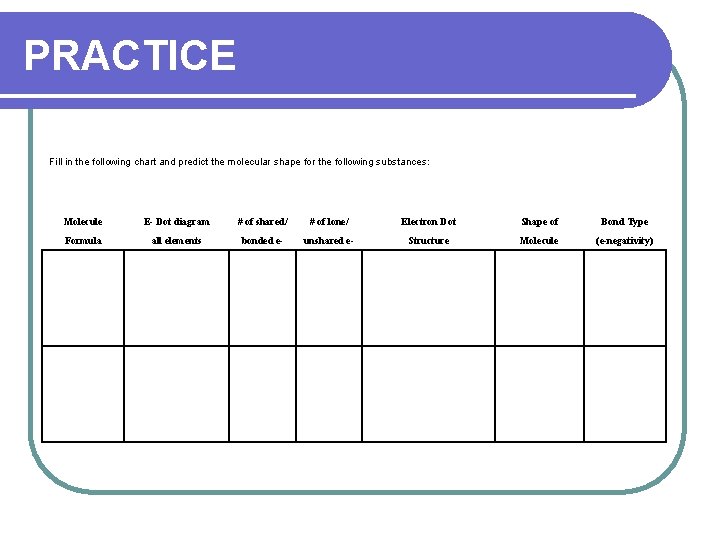
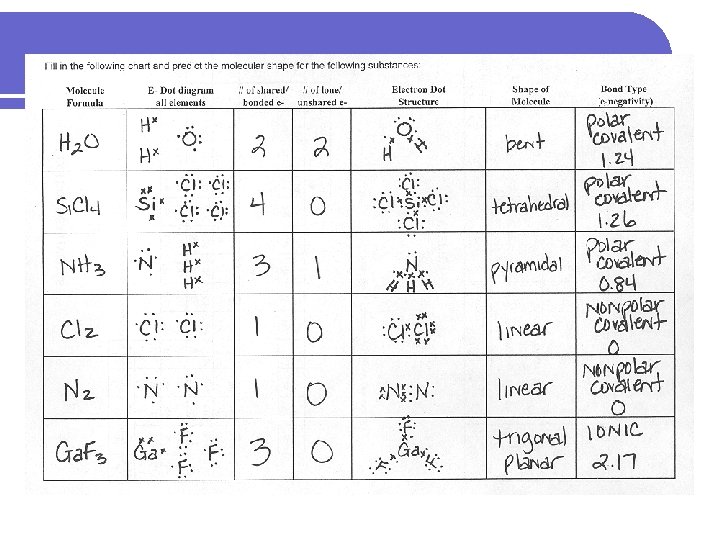
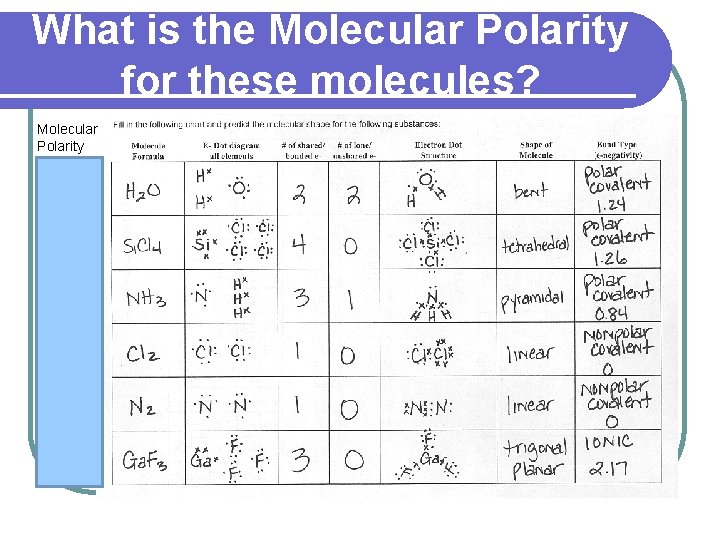
PRACTICE Fill in the following chart and predict the molecular shape for the following substances: Molecule E- Dot diagram # of shared/ # of lone/ Electron Dot Shape of Bond Type Formula all elements bonded e- unshared e- Structure Molecule (e-negativity)

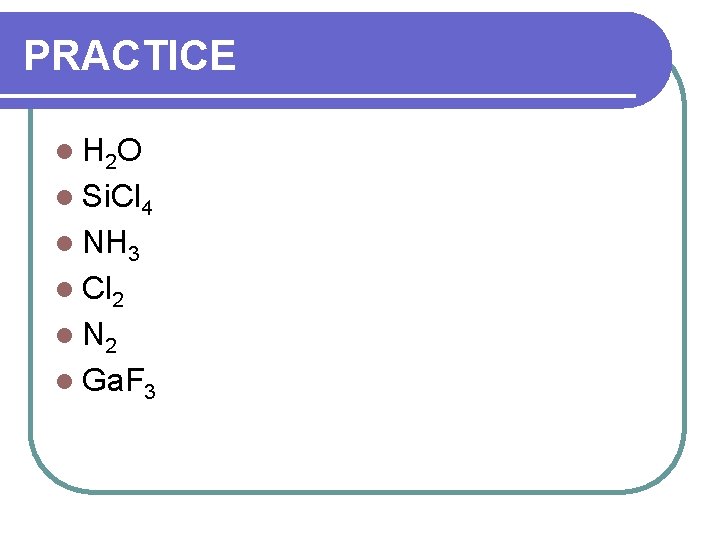
PRACTICE l H 2 O l Si. Cl 4 l NH 3 l Cl 2 l N 2 l Ga. F 3


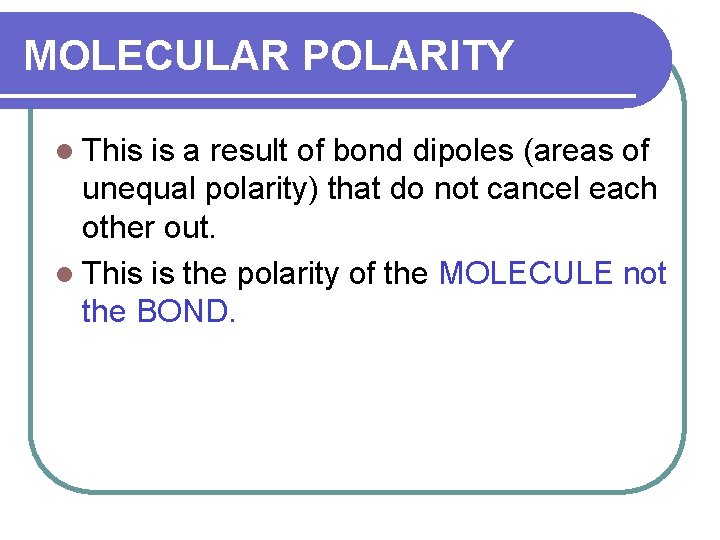
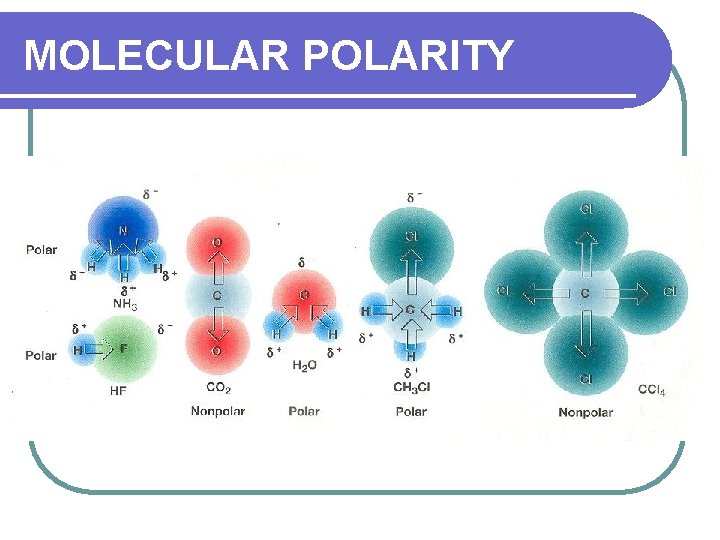
MOLECULAR POLARITY l This is a result of bond dipoles (areas of unequal polarity) that do not cancel each other out. l This is the polarity of the MOLECULE not the BOND.

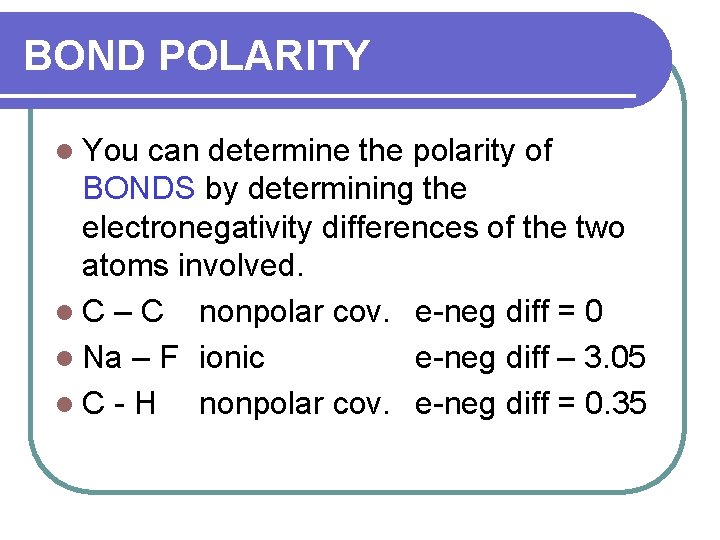
BOND POLARITY l You can determine the polarity of BONDS by determining the electronegativity differences of the two atoms involved. l C – C nonpolar cov. e-neg diff = 0 l Na – F ionic e-neg diff – 3. 05 l. C-H nonpolar cov. e-neg diff = 0. 35

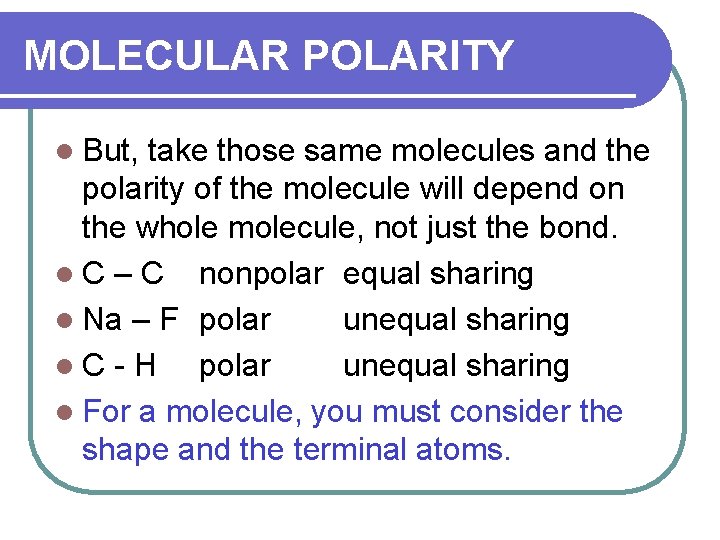
MOLECULAR POLARITY l But, take those same molecules and the polarity of the molecule will depend on the whole molecule, not just the bond. l C – C nonpolar equal sharing l Na – F polar unequal sharing l. C-H polar unequal sharing l For a molecule, you must consider the shape and the terminal atoms.

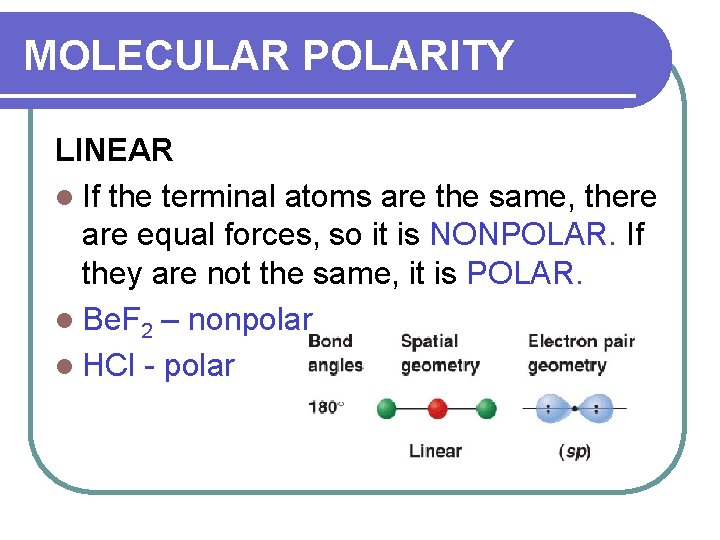
MOLECULAR POLARITY LINEAR l If the terminal atoms are the same, there are equal forces, so it is NONPOLAR. If they are not the same, it is POLAR. l Be. F 2 – nonpolar l HCl - polar

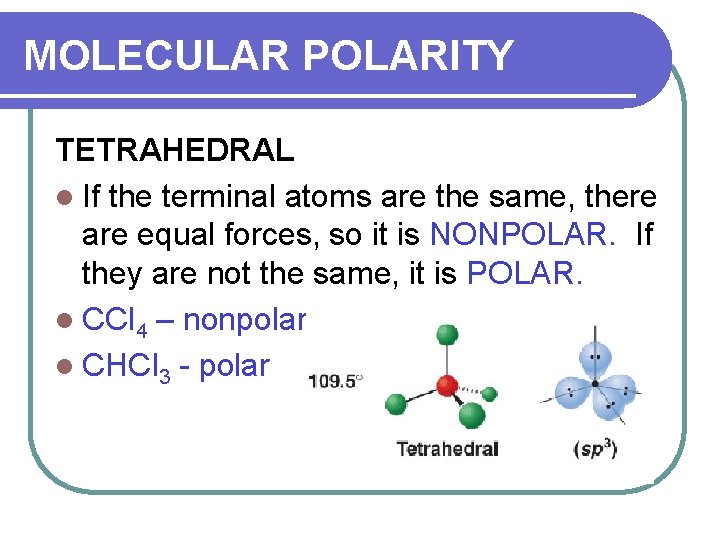
MOLECULAR POLARITY TETRAHEDRAL l If the terminal atoms are the same, there are equal forces, so it is NONPOLAR. If they are not the same, it is POLAR. l CCl 4 – nonpolar l CHCl 3 - polar

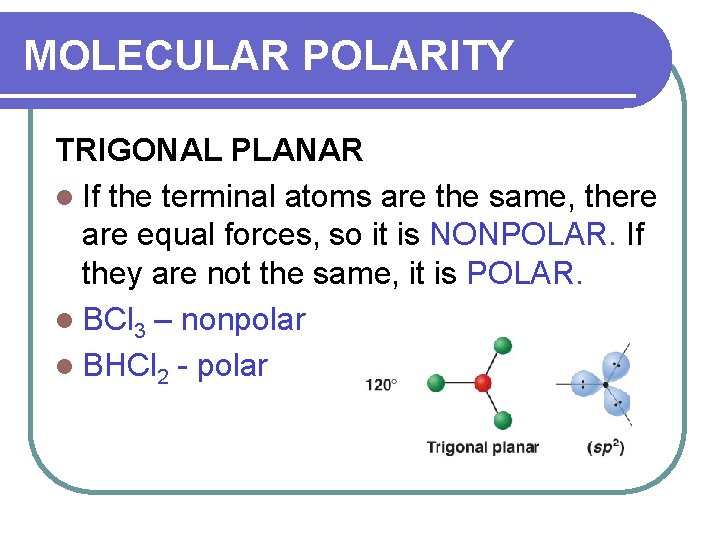
MOLECULAR POLARITY TRIGONAL PLANAR l If the terminal atoms are the same, there are equal forces, so it is NONPOLAR. If they are not the same, it is POLAR. l BCl 3 – nonpolar l BHCl 2 - polar

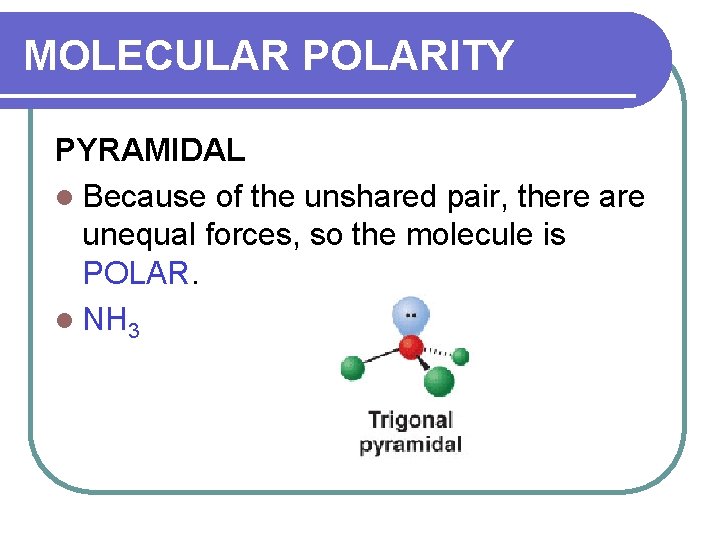
MOLECULAR POLARITY PYRAMIDAL l Because of the unshared pair, there are unequal forces, so the molecule is POLAR. l NH 3

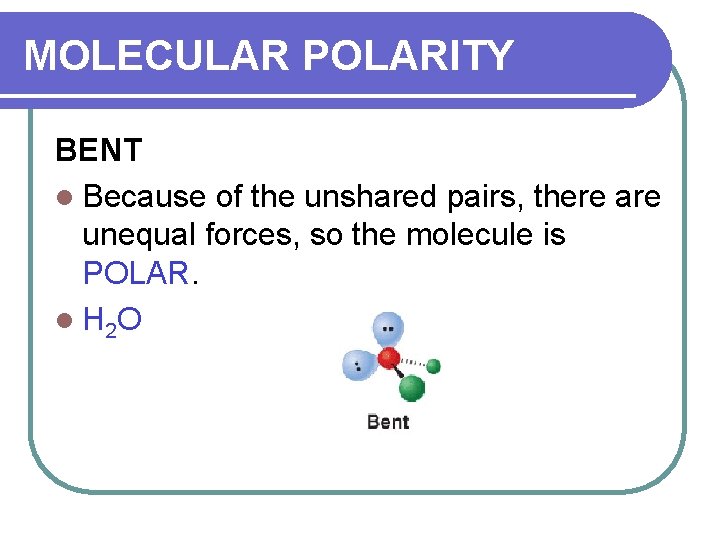
MOLECULAR POLARITY BENT l Because of the unshared pairs, there are unequal forces, so the molecule is POLAR. l H 2 O

MOLECULAR POLARITY

What is the Molecular Polarity for these molecules? Molecular Polarity

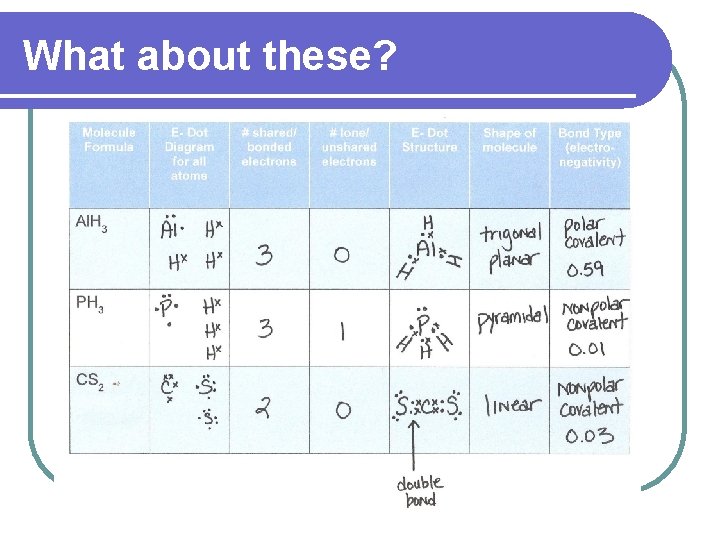
What about these?

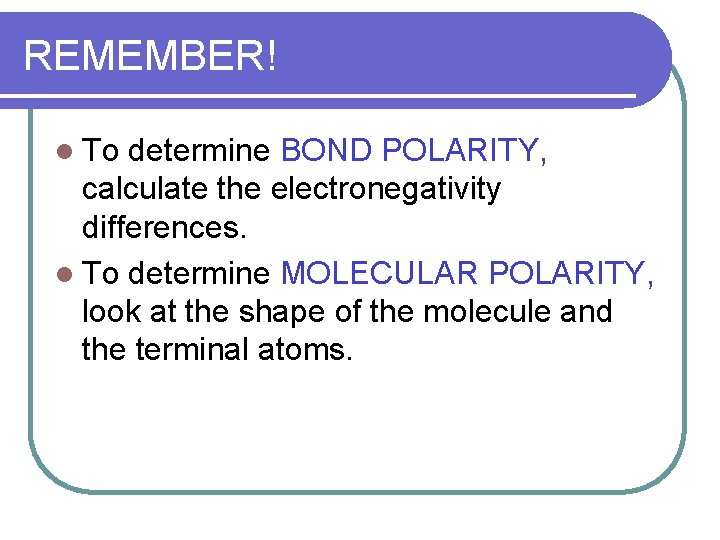
REMEMBER! l To determine BOND POLARITY, calculate the electronegativity differences. l To determine MOLECULAR POLARITY, look at the shape of the molecule and the terminal atoms.

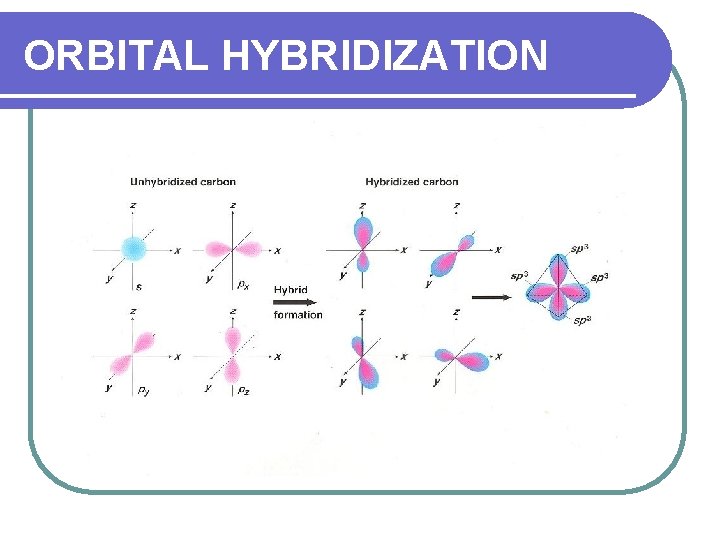
ORBITAL HYBRIDIZATION This is the mixing of atomic orbitals in an atom to generate a new set of atomic orbitals. S and p orbitals merge and there no longer are distinct orbitals. They merge to form sp orbitals.

ORBITAL HYBRIDIZATION

ORBITAL HYBRIDIZATION


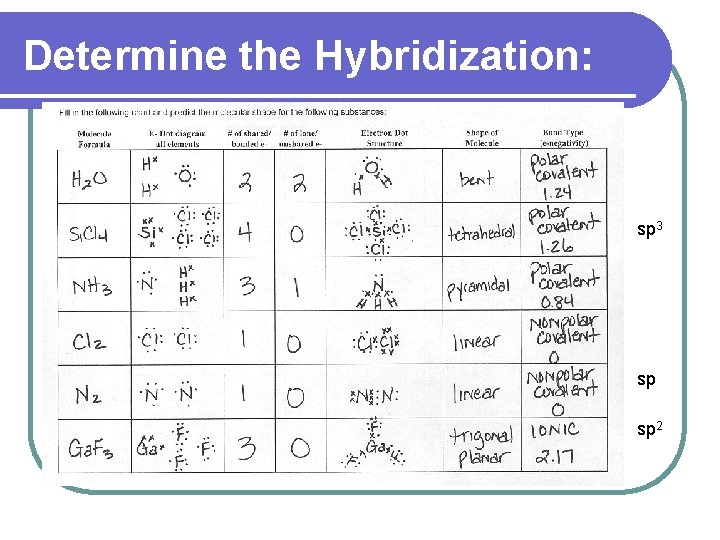
Determine the Hybridization: sp 3 sp sp 2

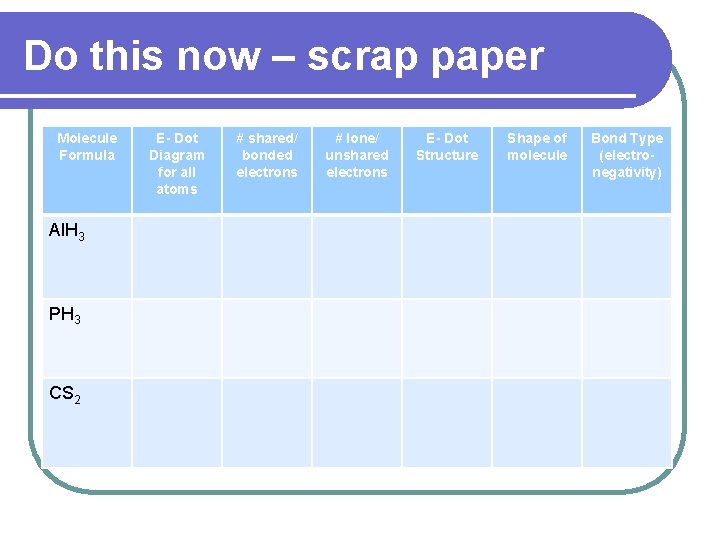
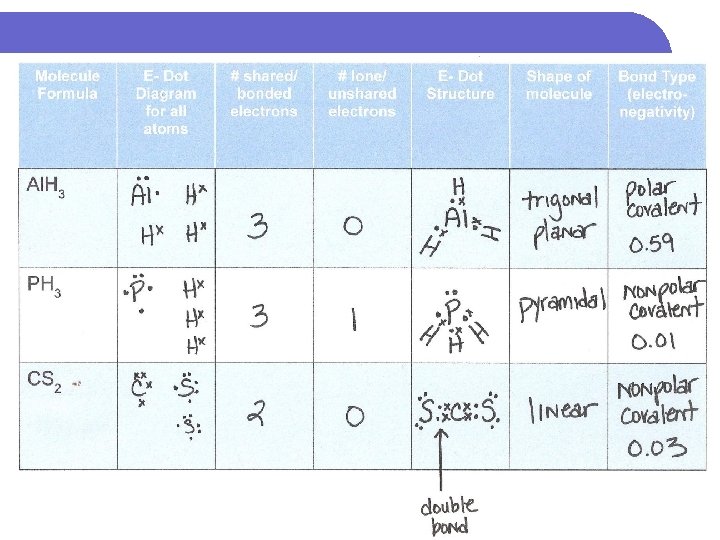
Do this now – scrap paper Molecule Formula Al. H 3 PH 3 CS 2 E- Dot Diagram for all atoms # shared/ bonded electrons # lone/ unshared electrons E- Dot Structure Shape of molecule Bond Type (electronegativity)
