Molecular Geometry and Polarity Molecule Covalent Bond shared
Molecular Geometry and Polarity
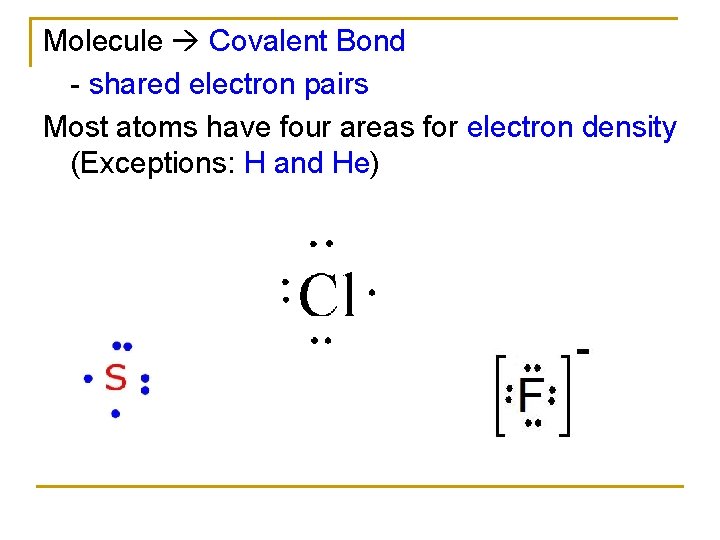
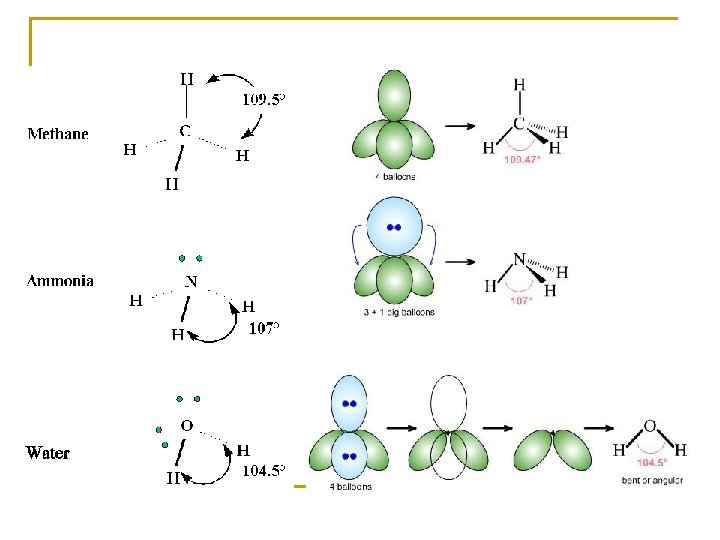
Molecule Covalent Bond - shared electron pairs Most atoms have four areas for electron density (Exceptions: H and He)
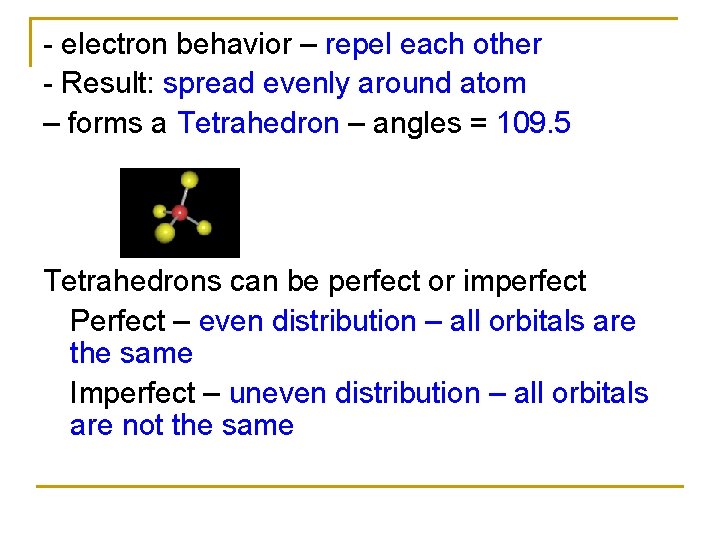
- electron behavior – repel each other - Result: spread evenly around atom – forms a Tetrahedron – angles = 109. 5 Tetrahedrons can be perfect or imperfect Perfect – even distribution – all orbitals are the same Imperfect – uneven distribution – all orbitals are not the same

Shape influenced by the types of orbitals Shared vs. Unshared Shared – between two atoms Unshared – around one atom q q q Shared are smaller because the 2 nuclei pull on the orbital making it have a narrower diameter Changes the angles between the bond axis in a molecule with a mixture of shared and unshared orbitals Shared orbitals are SMALLER than Unshared orbitals

n Ex: Dihydrogen Monoxide

Why? : electrons in a shared orbital pass between two atoms pulled on by two nuclei causes the orbital to be narrower Ex: pull on a balloon on both ends rather than one end RESULT: Unshared orbitals take up more space and push the shared orbitals closer together

Repulsion Hierarchy: unshared and unshared > unshared and shared > shared and shared
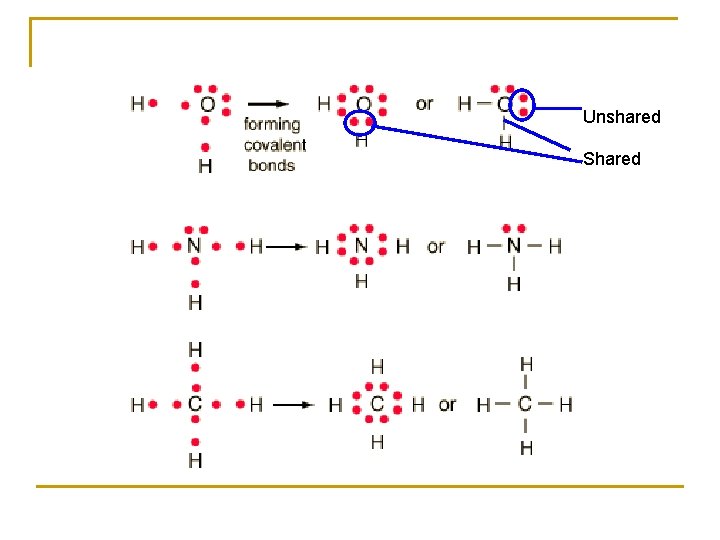
Unshared Shared
Importance of Bond Angles - influences the interaction of molecules - lead to the difference of a molecule being a solid, a liquid or a gas at room temperature and how it will react with other substances
Molecular Interactions Intramolecular Bonds - covalent bonds between two atoms Intermolecular Bonds - weak attractive forces between two molecules - the stronger an intermolecular bond – the more a substance will bond together
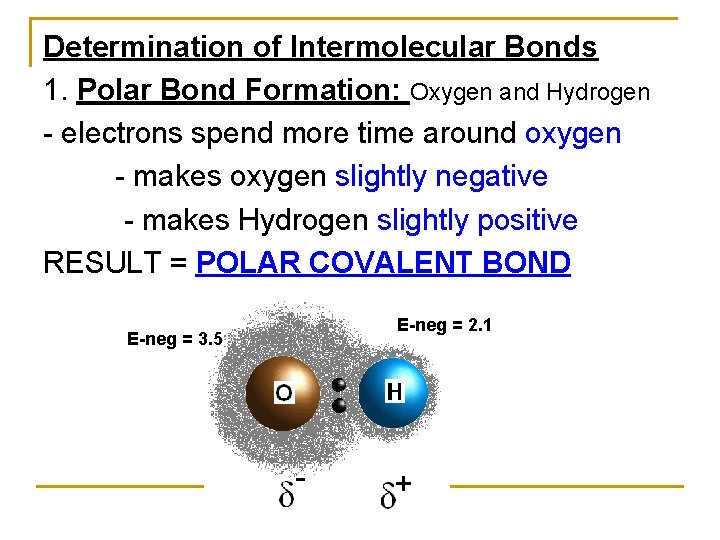
Determination of Intermolecular Bonds 1. Polar Bond Formation: Oxygen and Hydrogen - electrons spend more time around oxygen - makes oxygen slightly negative - makes Hydrogen slightly positive RESULT = POLAR COVALENT BOND E-neg = 3. 5 E-neg = 2. 1
- covalent bond that results from the unequal sharing of electrons - result is a bond with a positive and negative end (poles) - called a dipole moment - may give the molecule distinct poles of positive and negative charge if the charges are asymmetrically balanced
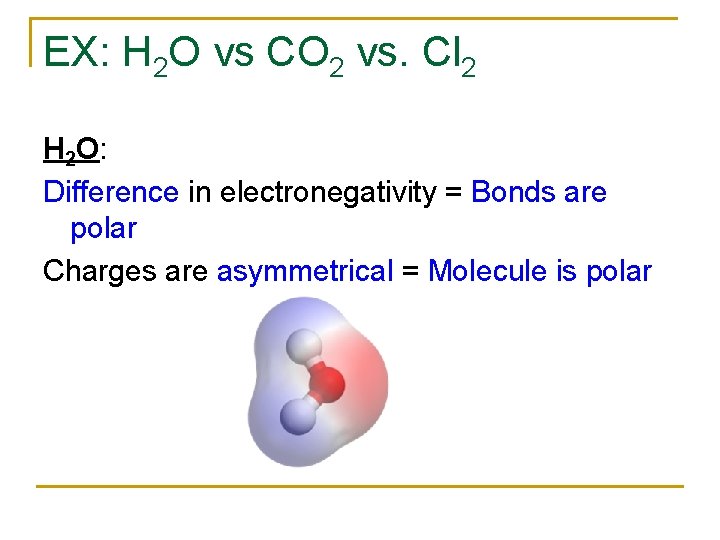
EX: H 2 O vs CO 2 vs. Cl 2 H 2 O: Difference in electronegativity = Bonds are polar Charges are asymmetrical = Molecule is polar
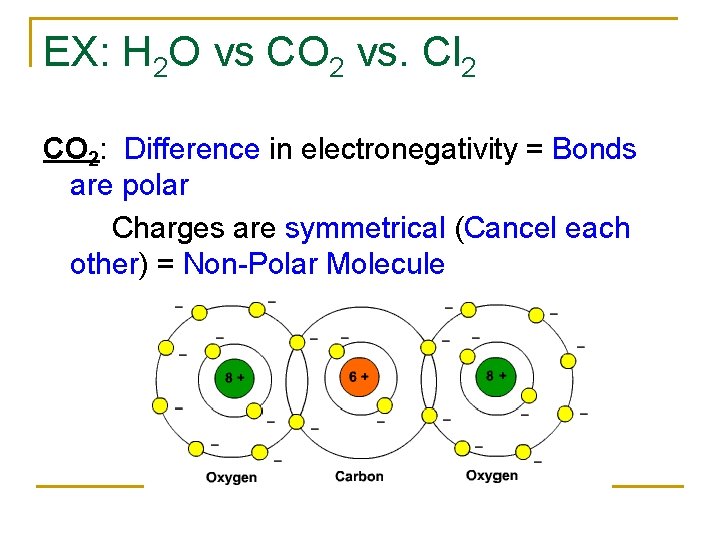
EX: H 2 O vs CO 2 vs. Cl 2 CO 2: Difference in electronegativity = Bonds are polar Charges are symmetrical (Cancel each other) = Non-Polar Molecule
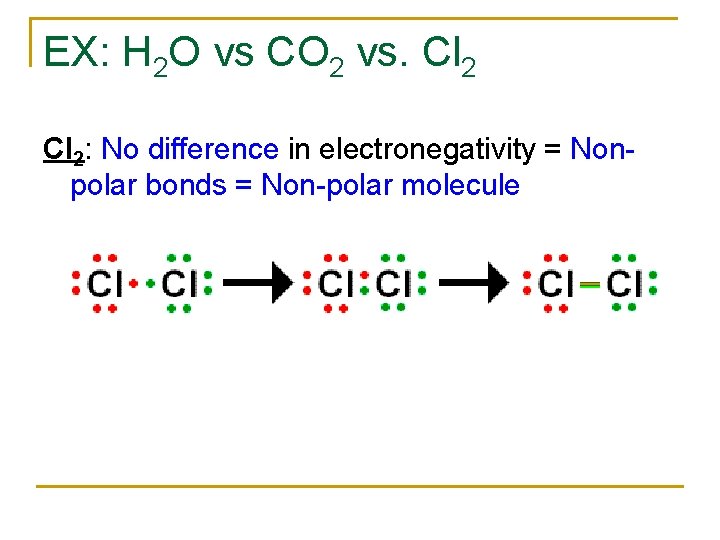
EX: H 2 O vs CO 2 vs. Cl 2: No difference in electronegativity = Nonpolar bonds = Non-polar molecule
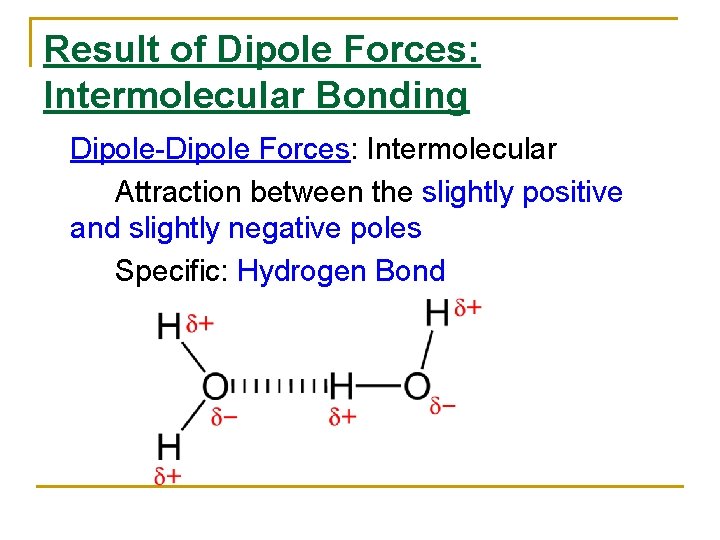
Result of Dipole Forces: Intermolecular Bonding Dipole-Dipole Forces: Intermolecular Attraction between the slightly positive and slightly negative poles Specific: Hydrogen Bond

Importance of Polarity 1. Binds molecules together Stronger = More Solid 2. Separation of Substances = FRACTIONATION - Chromatography - can determine the chemical make up of a mixture
Chromatography n n Animation II

3. Characteristics of Water a. Cohesion: water sticking to itself – surface tension

Characteristics of Water Adhesion: water sticking to other things – capillary action
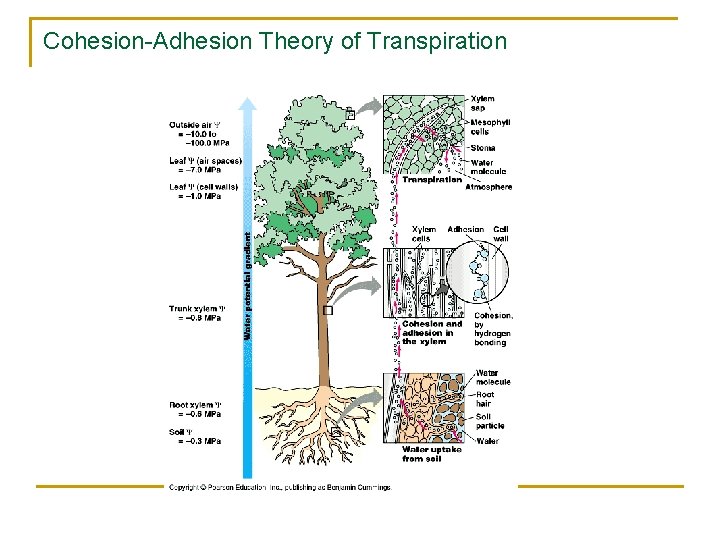
Cohesion-Adhesion Theory of Transpiration

c. High specific heat – Specific Heat – amount of heat a substance can absorb before it changes temperature High Specific Heat of Water (4. 186 J/go. C) - Ethanol = 2. 46 J/go. C Result: Climate Control – water heats and cools slowly which keeps land masses near bodies of water more temperate Evaporative Cooling – sweating – it takes a lot heat to cause the water to evaporate – heat is lost when the water leaves

Less dense as a solid – as water cools the hydrogen bonds between the molecules get stronger making the molecule assume a strict lattice structure – in this form the water molecules are more spread out and have a greater volume - equal mass over a greater volume = less dense ice floats - good for aquatic biomes – prevents bodies of water from freezing solid happy fish d.
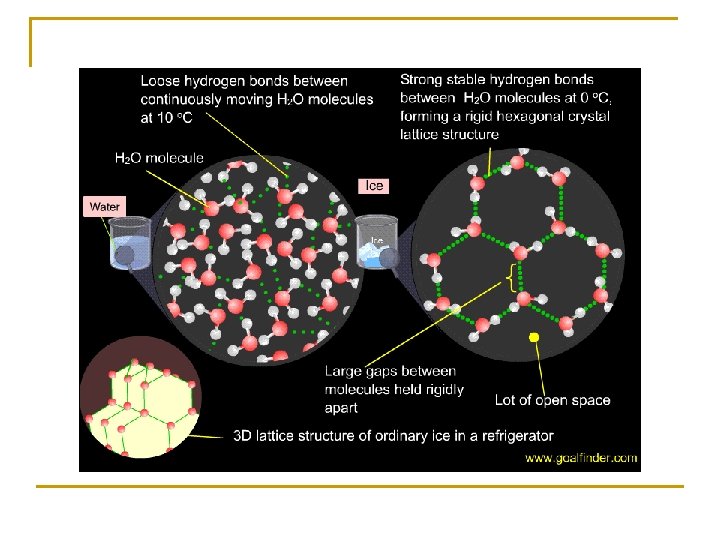

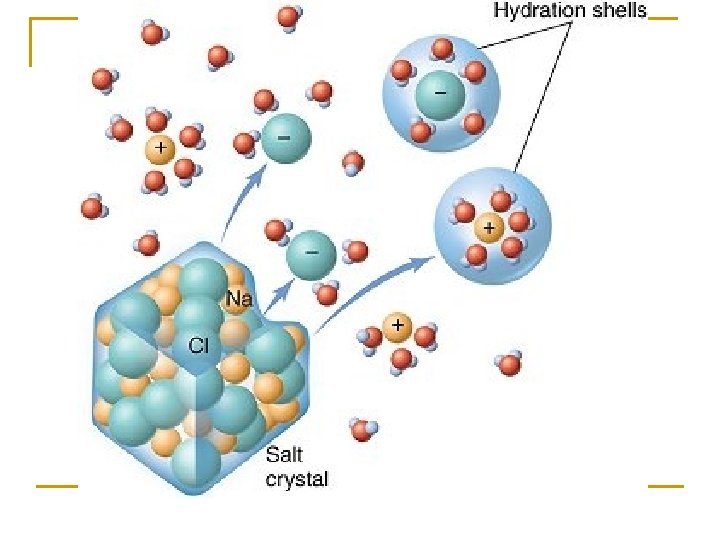
e. Solvent – water breaks apart substances – good for chemical reactions because the atoms can interact more easily if they are spread out – very important for the metabolism of cells -
- Slides: 28