Molecular Formulas A molecular formula is either the




- Slides: 5

Molecular Formulas A molecular formula is either the same as an empirical formula or it is a simple whole number multiple of its empirical formula.

Determining Molecular Formulas Calculate the molecular formula of a compound whose molar mass is 60. 0 g/mol and has an empirical formula of CH 4 N. What you need: Empirical Formula Molar Mass Calculate the molar mass of the empirical formula C: 12. 0 g x 1 H: 1. 0 g x 4 N: 14. 0 g x 1 = 12. 0 g = 4. 0 g = 14. 0 g 30. 0 g/mol Molecular Formula Molar Mass = Multiple 60. 0 g/mol Empirical Formula Molar Mass 30. 0 g/mol Empirical Formula X Multiple = Molecular Formula CH 4 N x 2 =2 = C 2 H 8 N 2

Determining Molecular Formulas Find the molecular formula of ethylene glycol which has a molar mass of 62 g/mol and an empirical formula of CH 3 O. What you need: Empirical Formula Molar Mass Calculate the molar mass of the empirical formula C: 12. 0 g x 1 H: 1. 0 g x 3 O: 16. 0 g x 1 = 12. 0 g = 4. 0 g = 16. 0 g 31. 0 g/mol Molecular Formula Molar Mass = Multiple 62 g/mol Empirical Formula Molar Mass 31. 0 g/mol Empirical Formula X Multiple = Molecular Formula CH 3 O x 2 =2 = C 2 H 6 O 2

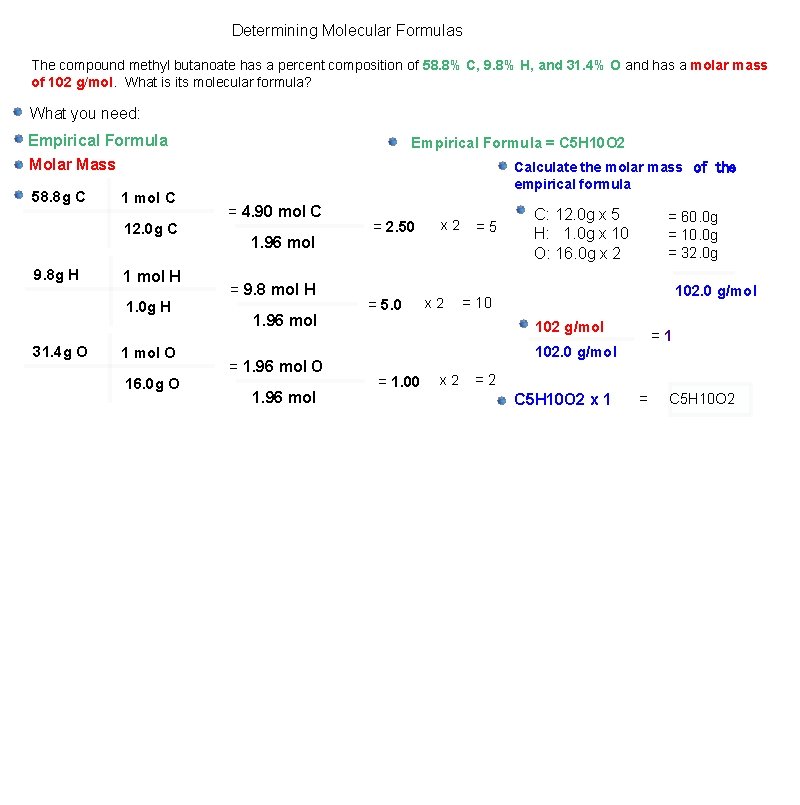
Determining Molecular Formulas The compound methyl butanoate has a percent composition of 58. 8% C, 9. 8% H, and 31. 4% O and has a molar mass of 102 g/mol. What is its molecular formula? What you need: Empirical Formula Molar Mass 58. 8 g C 1 mol C 12. 0 g C 9. 8 g H 1 mol H 1. 0 g H 31. 4 g O 1 mol O 16. 0 g O Empirical Formula = C 5 H 10 O 2 Calculate the molar mass of the empirical formula = 4. 90 mol C 1. 96 mol = 9. 8 mol H 1. 96 mol = 1. 96 mol O 1. 96 mol = 2. 50 = 5. 0 x 2 =5 C: 12. 0 g x 5 H: 1. 0 g x 10 O: 16. 0 g x 2 = 60. 0 g = 10. 0 g = 32. 0 g 102. 0 g/mol = 10 102 g/mol =1 102. 0 g/mol = 1. 00 x 2 =2 C 5 H 10 O 2 x 1 = C 5 H 10 O 2

Determining Molecular Formulas What is the molecular formula of a compound that has a percent composition of 94. 1% O and 5. 9% H and has a molar mass of 34 g/mol. What you need: Empirical Formula Molar Mass 5. 9 g H 1 mol H 1. 0 g H 94. 1 g O 1 mol C 16. 0 g O Empirical Formula = HO or OH Calculate the molar mass of the empirical formula = 5. 9 mol H 5. 88 mol H: 1. 0 g O: 16. 0 g = 1. 0 17. 0 g/mol = 5. 88 mol O 5. 88 mol = 1. 00 34 g/mol 17. 0 g/mol HO x 2 = H 2 O 2 or O 2 H 2 =2