Molecular Filtration National Air Filtration Association Technical Seminar








- Slides: 11

Molecular Filtration National Air Filtration Association Technical Seminar May, 2004 Paula Levasseur, CAFS Cameron/Great Lakes

Agenda • Contaminant Control • Removal Options – Adsorption – Chemisorption – Catalysis • Media Types • Definitions • Filter Options

Control Options • Source Control – Remove the contaminant source • Ventilation – Increase outdoor air • Removal Control – Remove contaminants via physical or chemical means

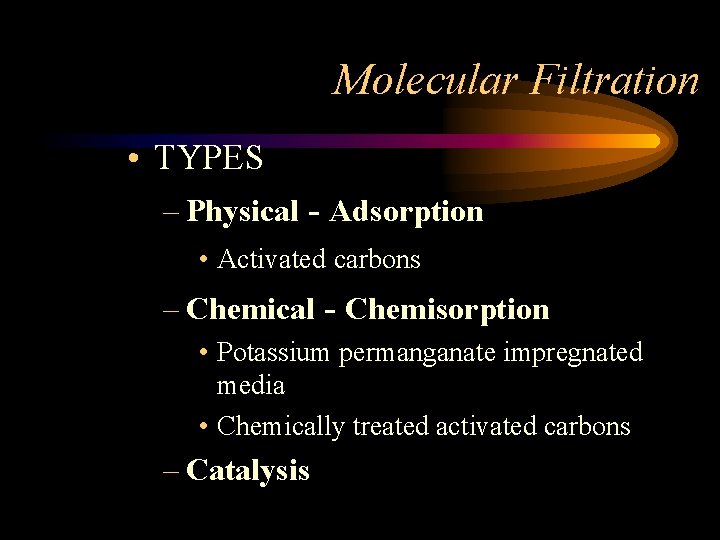
Molecular Filtration • TYPES – Physical - Adsorption • Activated carbons – Chemical - Chemisorption • Potassium permanganate impregnated media • Chemically treated activated carbons – Catalysis

Types • Adsorption - The process by which one substance is attracted and held onto the surface of another. – It is a surface phenomena. – Capacity is independent of particle size – Adsorption rate is inversely proportional to particle size. – High temperatures, may cause desorption. – Humidity has an adverse effect on adsorption – The contaminant being removed is called the adsorbate

Types • Chemisorption - The result of chemical reactions on and in the surface of the adsorbent. – Fairly specific and depends upon chemical nature of media and the contaminant – Irreversible & essentially instantaneous – Oxidation - changes harmful gases to harmless solids – Higher temperatures will increase the reaction rate in chemisorption – Humidity is favorable toward the reaction

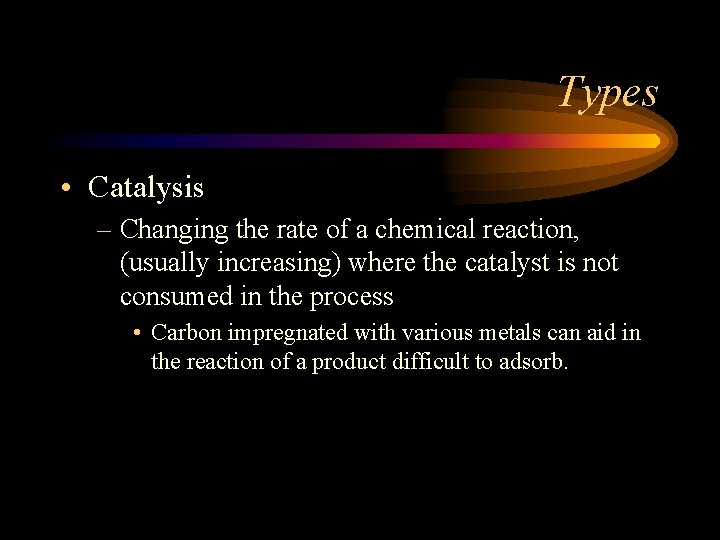
Types • Catalysis – Changing the rate of a chemical reaction, (usually increasing) where the catalyst is not consumed in the process • Carbon impregnated with various metals can aid in the reaction of a product difficult to adsorb.

Media Overview • Activated Carbon – adsorption – may be reactivated • Chemically Treated Carbon – chemisorption • Specific to a compound or family of compounds – not reversible • Potassium Permanganate – chemisorption • broad based oxidizer – not reversible

Definitions • RESIDENCE TIME – The time it takes air to cross a distance equal to the thickness of the filter without accounting for the resistance of the media through which it travels.

Definitions • REMOVAL EFFICIENCY – The fraction of the contaminant that, once in contact with the media, is removed by either physical or chemical means. • CAPACITY – The amount of contaminant the media is capable of removing. This determines the life of the filter.

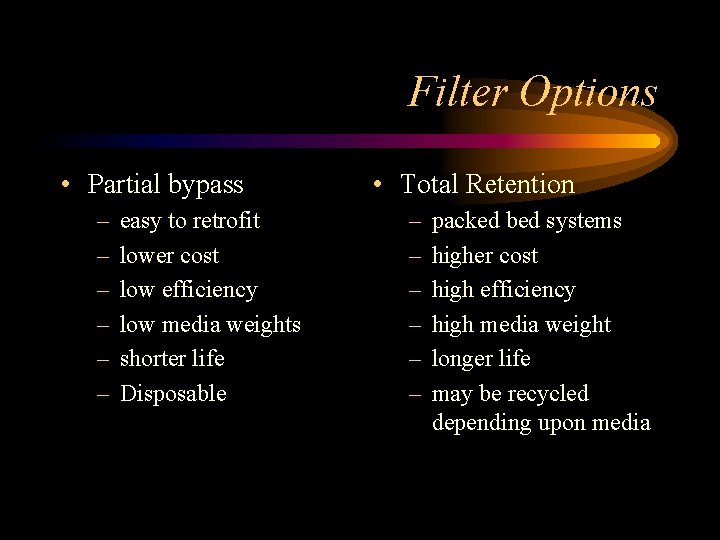
Filter Options • Partial bypass – – – easy to retrofit lower cost low efficiency low media weights shorter life Disposable • Total Retention – – – packed bed systems higher cost high efficiency high media weight longer life may be recycled depending upon media
 National air filtration association
National air filtration association National air filtration association
National air filtration association Specific cake resistance and filter medium resistance
Specific cake resistance and filter medium resistance Air higroskopis air kapiler dan air gravitasi
Air higroskopis air kapiler dan air gravitasi Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Nafa guide to air filtration pdf
Nafa guide to air filtration pdf Nafa guide to air filtration
Nafa guide to air filtration Nafa guide to air filtration
Nafa guide to air filtration Nafa filtration
Nafa filtration Nafa guide to air filtration
Nafa guide to air filtration