Molecular Compounds What are molecular compounds and how






- Slides: 19

Molecular Compounds What are molecular compounds and how are they different from ionic compounds?

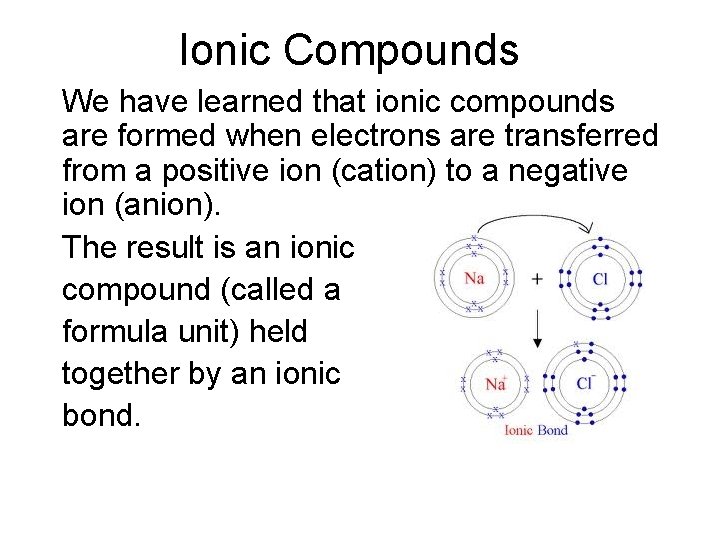
Ionic Compounds We have learned that ionic compounds are formed when electrons are transferred from a positive ion (cation) to a negative ion (anion). The result is an ionic compound (called a formula unit) held together by an ionic bond.

Hydrogen Atoms There is another way in which compounds can form. For example consider 2 hydrogen atoms. Each hydrogen atom has one electron. For the two hydrogen atoms to become stable, they must both gain an electron.

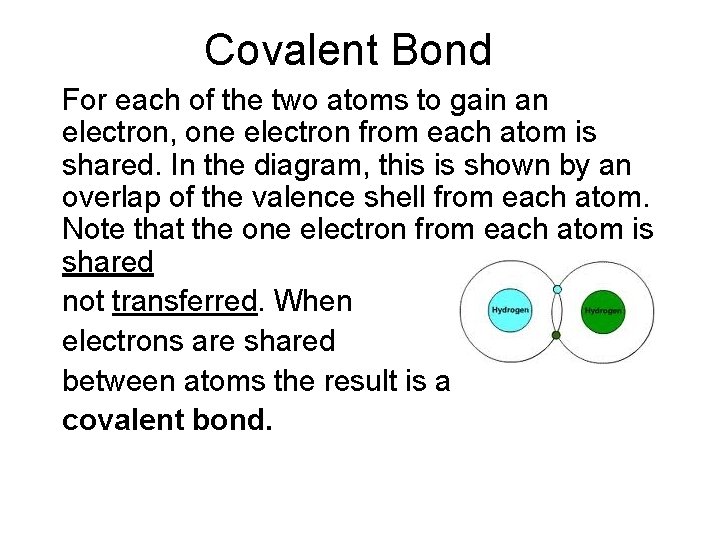
Covalent Bond For each of the two atoms to gain an electron, one electron from each atom is shared. In the diagram, this is shown by an overlap of the valence shell from each atom. Note that the one electron from each atom is shared not transferred. When electrons are shared between atoms the result is a covalent bond.

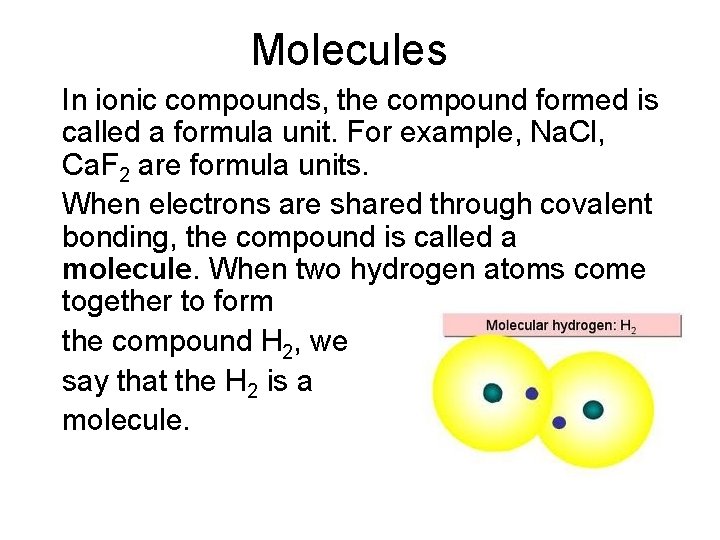
Molecules In ionic compounds, the compound formed is called a formula unit. For example, Na. Cl, Ca. F 2 are formula units. When electrons are shared through covalent bonding, the compound is called a molecule. When two hydrogen atoms come together to form the compound H 2, we say that the H 2 is a molecule.

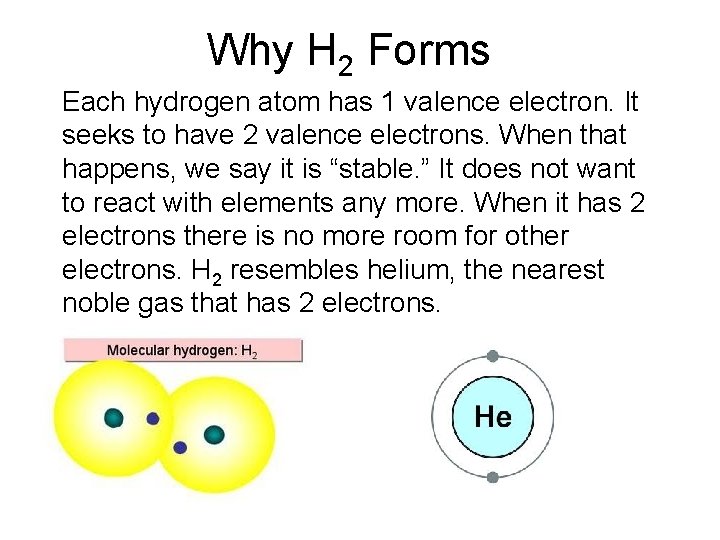
Why H 2 Forms Each hydrogen atom has 1 valence electron. It seeks to have 2 valence electrons. When that happens, we say it is “stable. ” It does not want to react with elements any more. When it has 2 electrons there is no more room for other electrons. H 2 resembles helium, the nearest noble gas that has 2 electrons.

Octet Rule When elements react with each other, they try to reach a “stable” state resembling the nearest noble gas. Except for helium with its 2 electrons, all noble gases have 8 valence electrons. The octet rule states that when elements react with each other, they try to do so in such a way that they have 8 electrons in the outer shell.

Cl 2 Molecule Another example of a molecule where covalent bonding takes place is chlorine. Each chlorine atom has 7 valence electrons. To form a molecule, each atom shares one electron with the other. Notice in this diagram that the valence shell from each atom overlaps with the other. Two chlorine atoms bond together to make a chlorine molecule: Cl 2. Note that 1 pair of electrons is being shared. This allows each chlorine to have 8 valence electrons.

Diatomic Molecules When two atoms come together to share electrons, the result is a diatomic molecule. The chart below shows examples of diatomic molecules.

Methane Carbon has 4 valence electrons. It needs 4 more to have a complete outer shell of 8. Hydrogen has 1 valence electron. It needs 1 more for its outer shell to be complete. The best arrangement for both is to have 4 H atoms and 1 C atom. Each H atom shares 1 electron, and the C shares its 4 electrons with the 4 H atoms. The molecule is CH 4, also called methane. Note that 4 pairs of electrons are being shared. We say that 4 covalent bonds are formed.

Water Oxygen has 6 valence electrons. It needs 2 more to complete its outer shell. Hydrogen has 1 valence electron and needs 1 more. Therefore two H atoms share 1 electron each with the O atom, and the O atom shares 1 of its electrons with each H atom. Note that 2 pairs of electrons are being shared. We say 2 covalent bonds are formed.

Ammonia Nitrogen has 5 valence electrons. It needs 3 more to complete its outer shell. Hydrogen has 1 valence electron and needs 1 more. Therefore three H atoms share 1 electron each with the N atom, and the N atom shares 1 of its electrons with each H atom. Note that three pairs of electrons are being shared. We say 3 covalent bonds are formed.

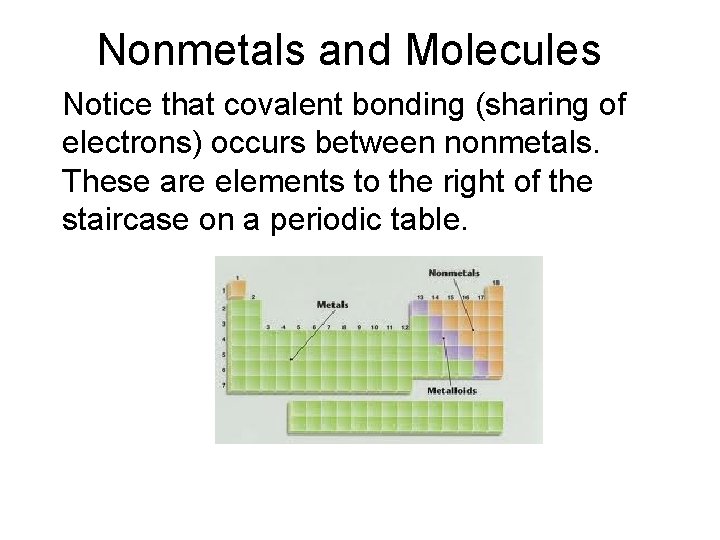
Nonmetals and Molecules Notice that covalent bonding (sharing of electrons) occurs between nonmetals. These are elements to the right of the staircase on a periodic table.

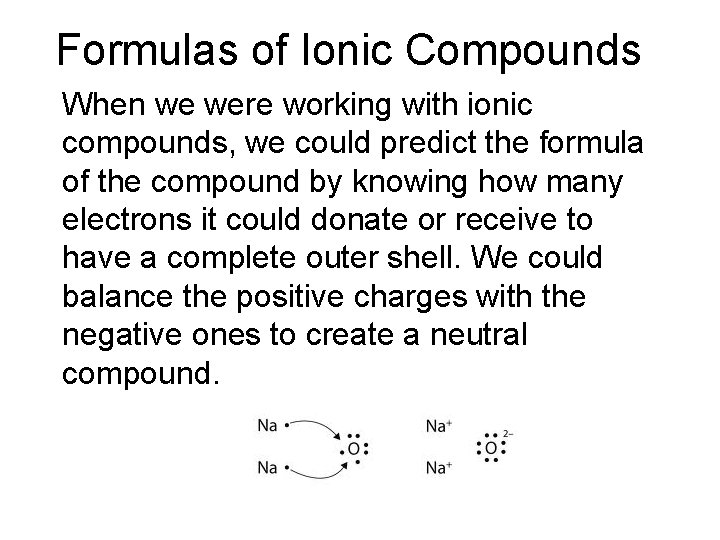
Formulas of Ionic Compounds When we were working with ionic compounds, we could predict the formula of the compound by knowing how many electrons it could donate or receive to have a complete outer shell. We could balance the positive charges with the negative ones to create a neutral compound.

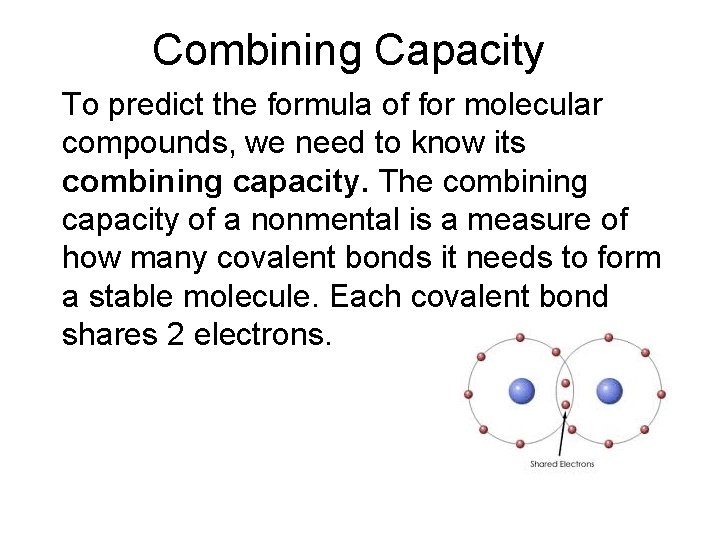
Combining Capacity To predict the formula of for molecular compounds, we need to know its combining capacity. The combining capacity of a nonmental is a measure of how many covalent bonds it needs to form a stable molecule. Each covalent bond shares 2 electrons.

Combining Capacity List The following chart shows the combining capacities of some of the nonmetals. This is very similar to what the charge of the negative ions would be.

Writing Molecular Formulas The following shows how to determine the formula of a molecular compound when the combining capacities are known.

Checklist 1. 2. 3. 4. 5. 6. 7. 8. Explain how covalent bonds and molecules form. Describe the difference between ionic bonds and covalent bonds by referring to the “types” of atoms that form these kinds of bonds, and whether electrons are shared or transferred. Draw Bohr models of molecular compounds. Define the term molecule and recognize a molecule when a formula or other description is given. List the molecules that are diatomic. Describe what elements on the periodic table, when combined, form molecules. Explain what is meant by the octet rule and how it applies to the formation of compounds. Explain what is meant by “combining capacity” and how this can be used to predict the chemical formula of a molecule.

Practice