Molecular Compounds SNC 2 D Covalent bonding Thus
Molecular Compounds SNC 2 D

Covalent bonding • Thus far we have looked at when atoms bond due to the transfer of electrons • An ionic bond forms when an atom has a greater attraction for e–s than a second atom • However, if two atoms have approximately the same pull on electrons, they share the electrons (forming a “covalent” bond) • BUT HOW DO WE KNOW? ? ?
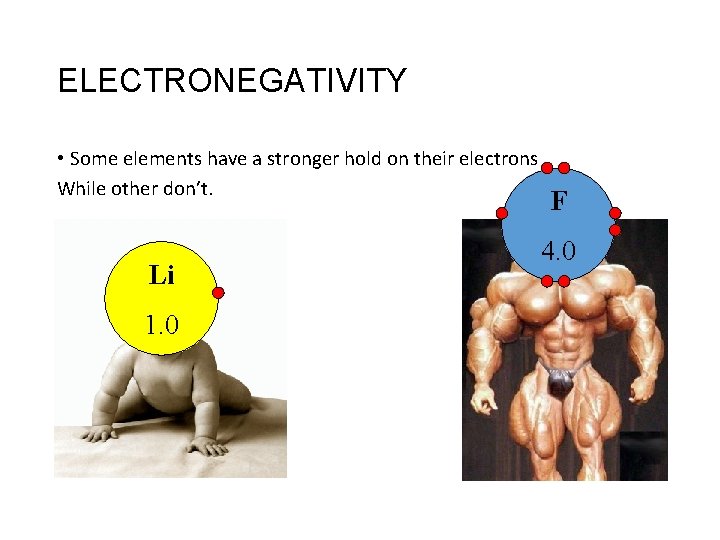
ELECTRONEGATIVITY • Some elements have a stronger hold on their electrons While other don’t. Li 1. 0 F 4. 0
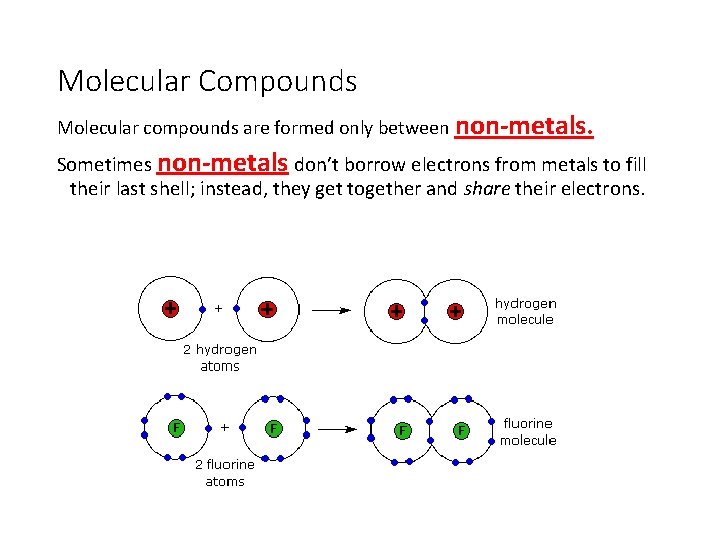
Molecular Compounds Molecular compounds are formed only between non-metals. Sometimes non-metals don’t borrow electrons from metals to fill their last shell; instead, they get together and share their electrons.
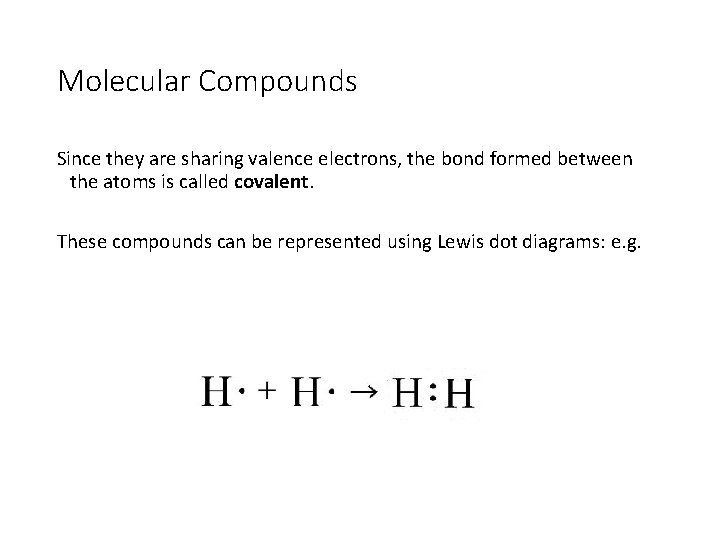
Molecular Compounds Since they are sharing valence electrons, the bond formed between the atoms is called covalent. These compounds can be represented using Lewis dot diagrams: e. g.
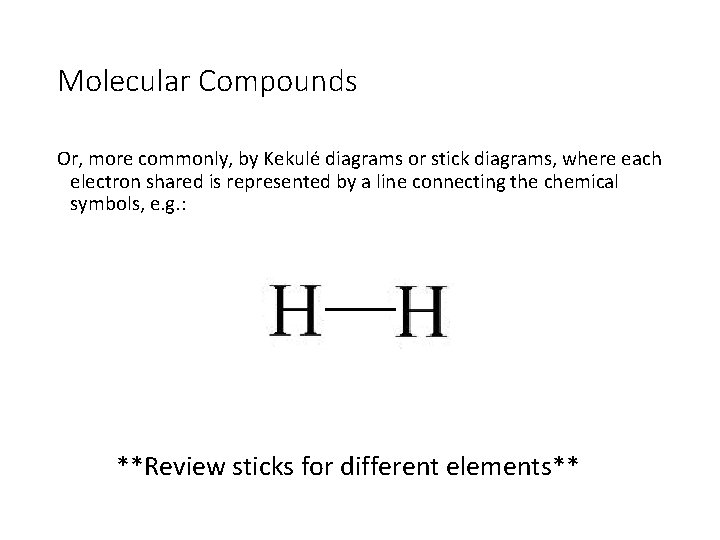
Molecular Compounds Or, more commonly, by Kekulé diagrams or stick diagrams, where each electron shared is represented by a line connecting the chemical symbols, e. g. : **Review sticks for different elements**
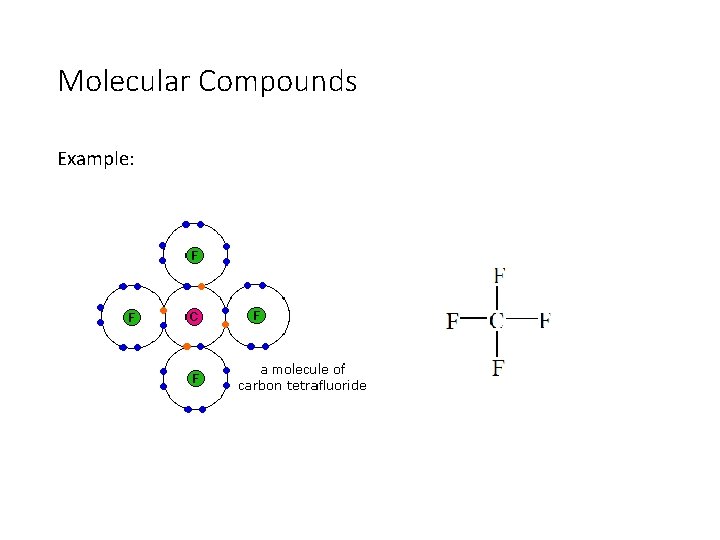
Molecular Compounds Example:
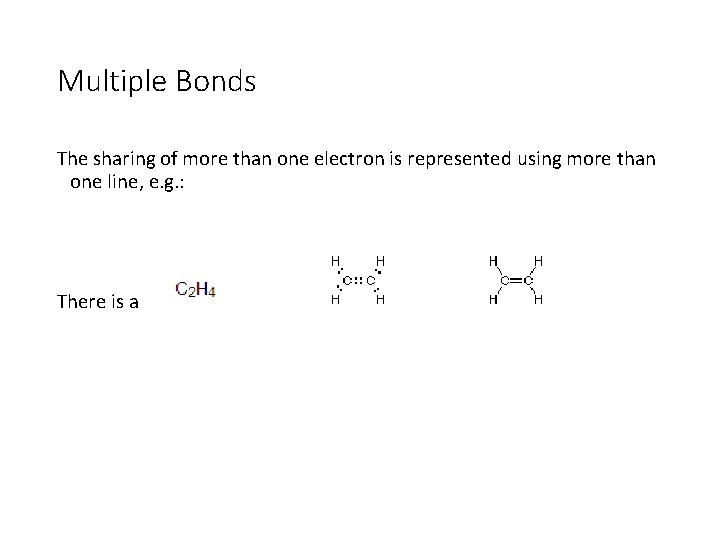
Multiple Bonds The sharing of more than one electron is represented using more than one line, e. g. : There is a double bond between the carbon atoms.
Naming Molecular Compounds The elements in the name are given prefixes corresponding to the subscripts (number of atoms) and the second element is given the suffix “-ide. ” e. g. CO 2 is carbon dioxide

The Prefixes • 1 Mono-* • 2 Di • 3 Tri • 4 Tetra • 5 Penta • 6 Hexa • 7 Hepta • 8 Octa • Mono is only used when the second element is oxygen* • Eg. CO Carbon monoxide
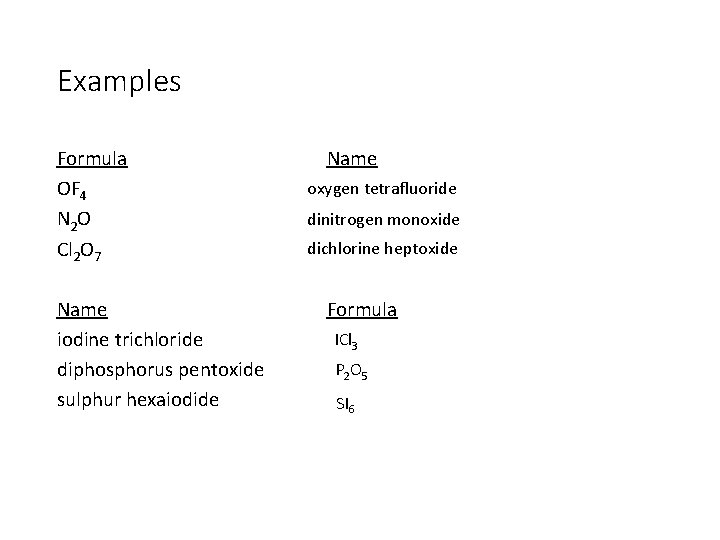
Examples Formula OF 4 N 2 O Cl 2 O 7 Name iodine trichloride diphosphorus pentoxide sulphur hexaiodide Name oxygen tetrafluoride dinitrogen monoxide dichlorine heptoxide Formula ICl 3 P 2 O 5 SI 6

Non-Conventional Names Some compounds are more commonly known by other names. e. g. NH 3 CH 4 H 2 O ammonia methane water
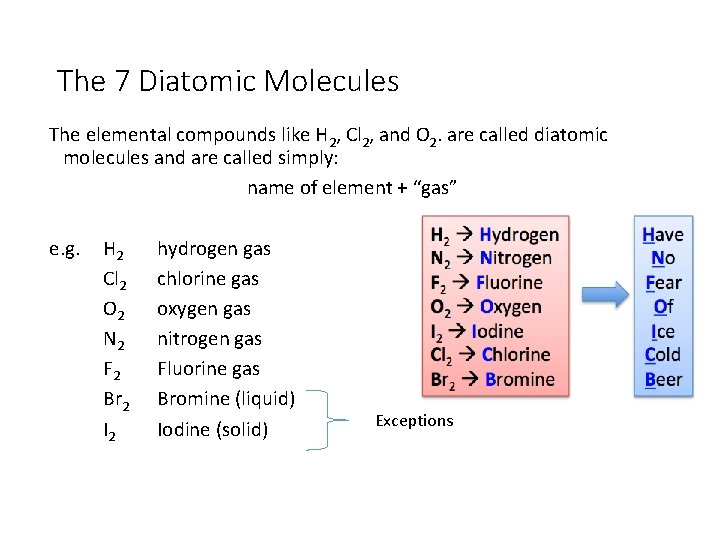
The 7 Diatomic Molecules The elemental compounds like H 2, Cl 2, and O 2. are called diatomic molecules and are called simply: name of element + “gas” e. g. H 2 Cl 2 O 2 N 2 F 2 Br 2 I 2 hydrogen gas chlorine gas oxygen gas nitrogen gas Fluorine gas Bromine (liquid) Iodine (solid) Exceptions
- Slides: 13