Molecular Compounds Ionic vs Molecular Crystalline May be
Molecular Compounds
Ionic vs. Molecular • Crystalline _______ • May be _____ (sugar), • ______(gasoline) • ______(carbon dioxide)
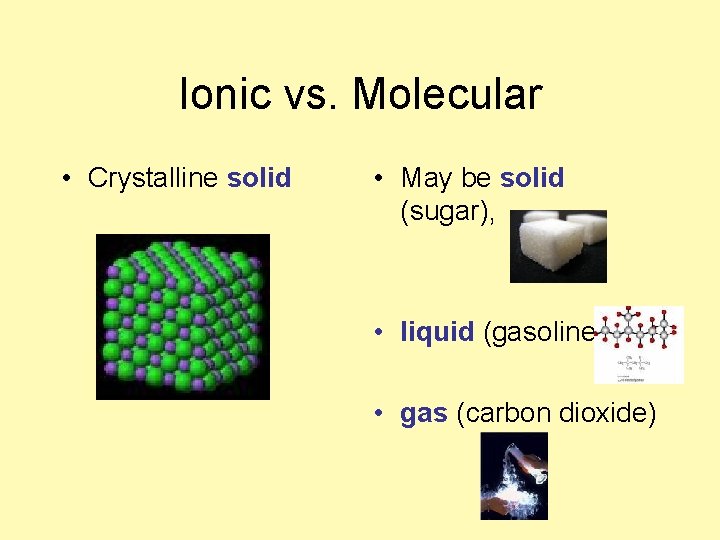
Ionic vs. Molecular • Crystalline solid • May be solid (sugar), • liquid (gasoline) • gas (carbon dioxide)
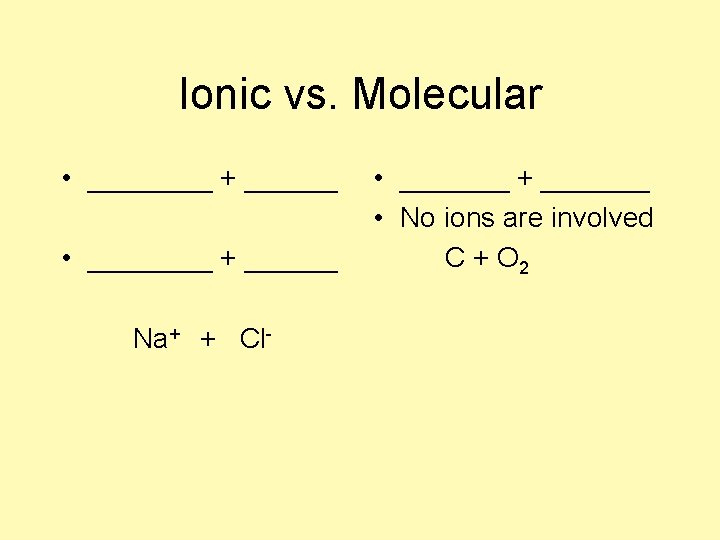
Ionic vs. Molecular • ________ + ______ Na+ + Cl- • _______ + _______ • No ions are involved C + O 2
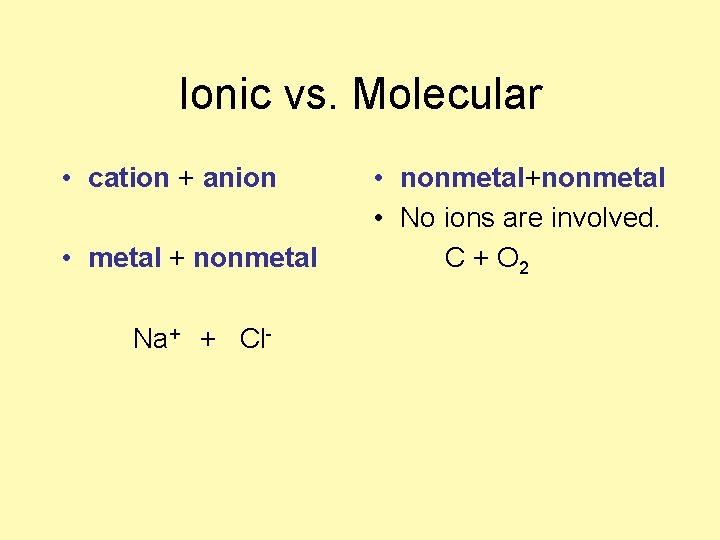
Ionic vs. Molecular • cation + anion • metal + nonmetal Na+ + Cl- • nonmetal+nonmetal • No ions are involved. C + O 2
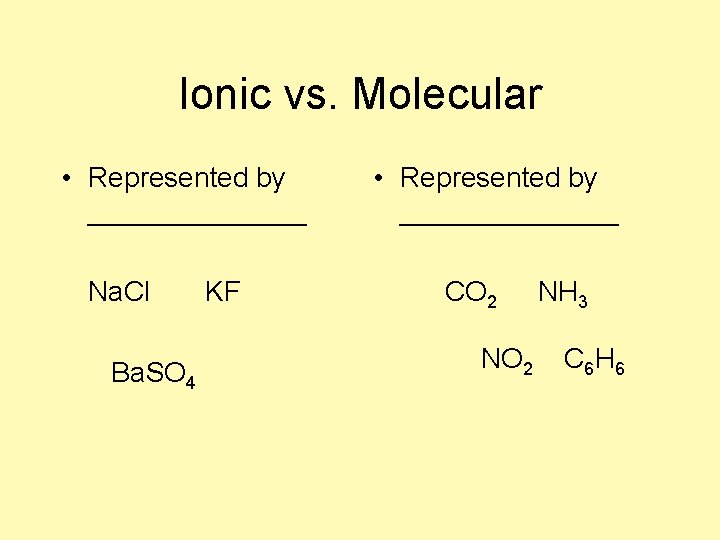
Ionic vs. Molecular • Represented by _______ Na. Cl Ba. SO 4 KF • Represented by _______ CO 2 NH 3 C 6 H 6
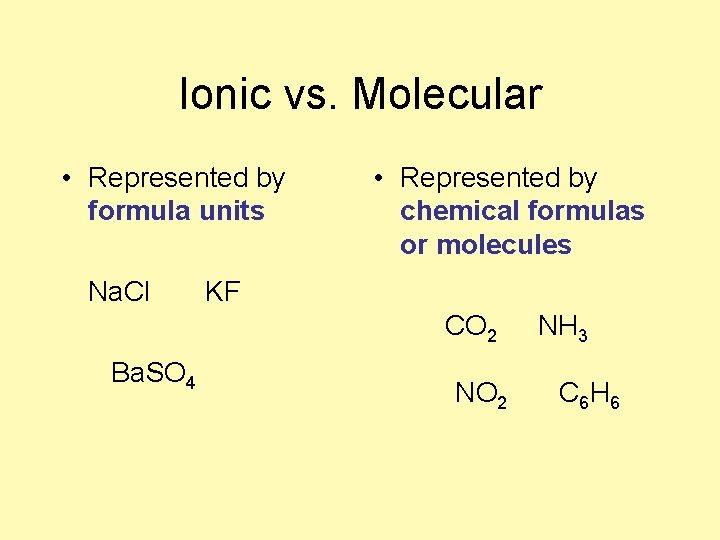
Ionic vs. Molecular • Represented by formula units Na. Cl • Represented by chemical formulas or molecules KF CO 2 Ba. SO 4 NO 2 NH 3 C 6 H 6
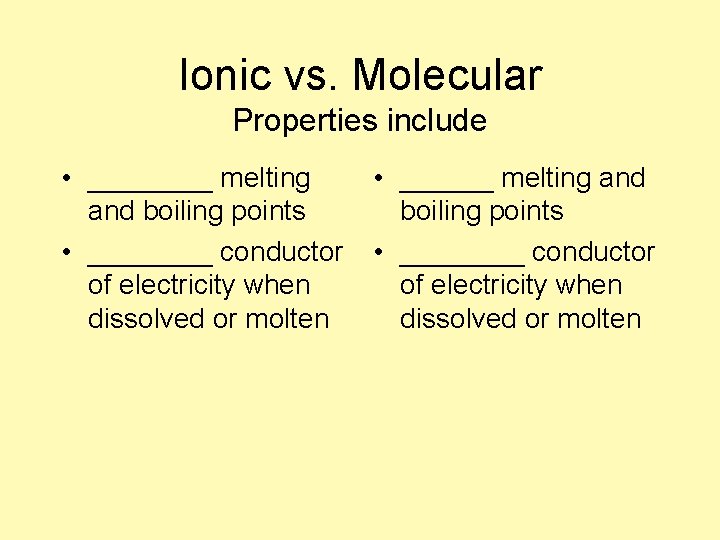
Ionic vs. Molecular Properties include • ____ melting and boiling points • ____ conductor of electricity when dissolved or molten • ______ melting and boiling points • ____ conductor of electricity when dissolved or molten
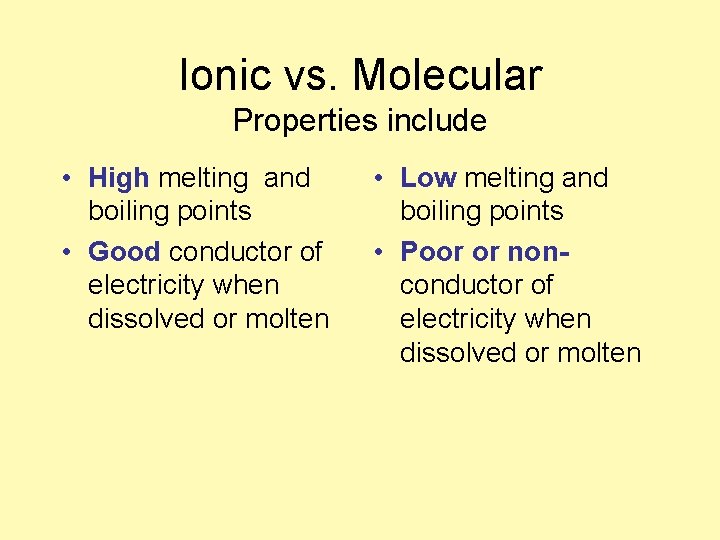
Ionic vs. Molecular Properties include • High melting and boiling points • Good conductor of electricity when dissolved or molten • Low melting and boiling points • Poor or nonconductor of electricity when dissolved or molten
Ionic vs. Molecular • Form by ____ of electrons Na Cl • Form by ____ of electrons O C O
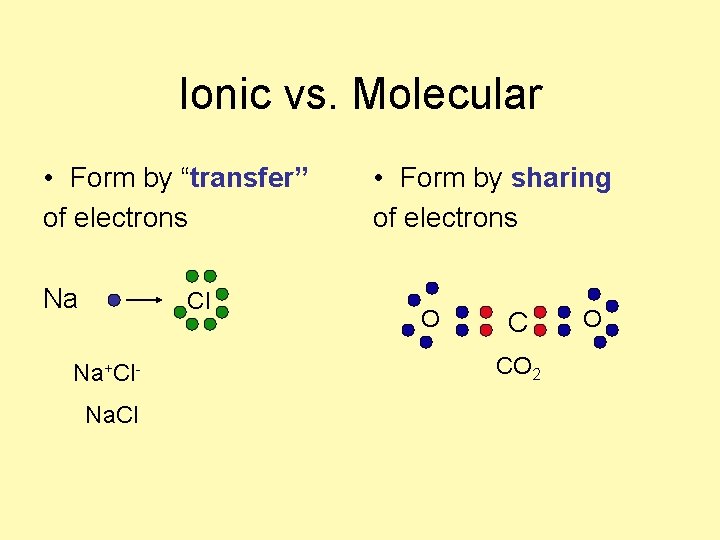
Ionic vs. Molecular • Form by “transfer” of electrons Na Cl Na+Cl. Na. Cl • Form by sharing of electrons O C CO 2 O
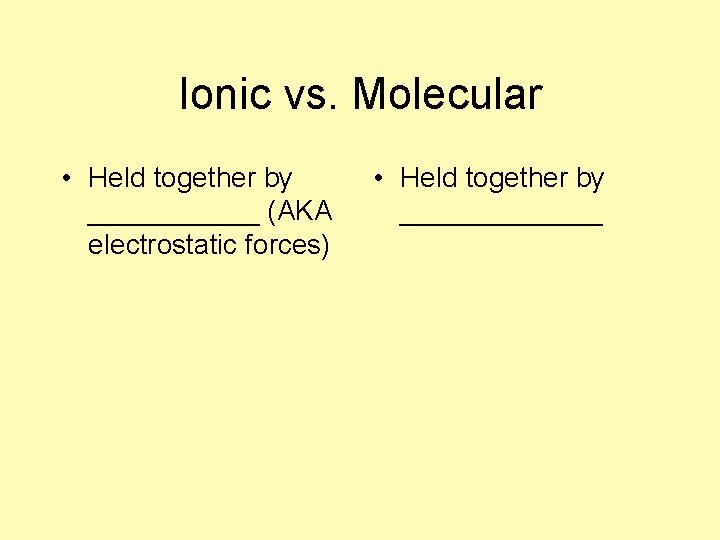
Ionic vs. Molecular • Held together by ______ (AKA electrostatic forces) • Held together by _______
Ionic vs. Molecular • Held together by ionic bonds (AKA electrostatic forces) • Held together by covalent bonds

Diatomic Molecules
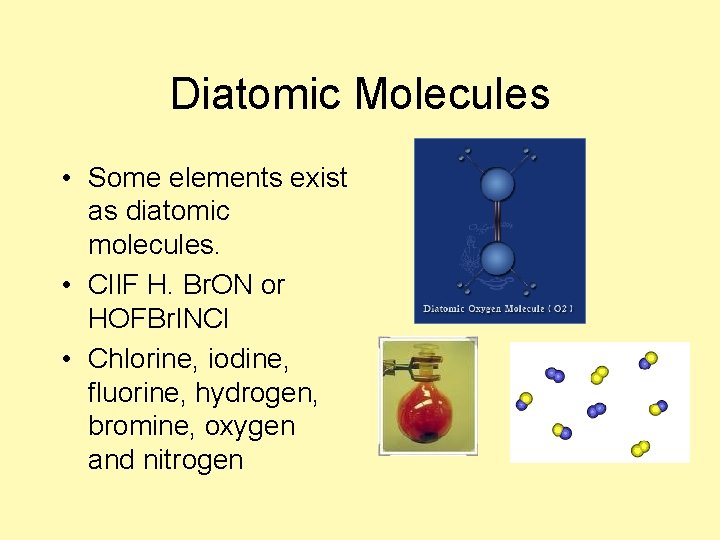
Diatomic Molecules • Some elements exist as diatomic molecules. • Cl. IF H. Br. ON or HOFBr. INCl • Chlorine, iodine, fluorine, hydrogen, bromine, oxygen and nitrogen
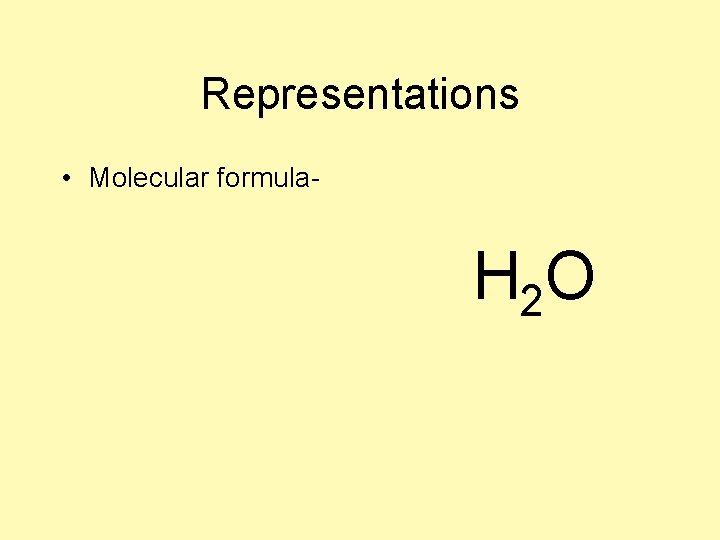
Representations • Molecular formula- H 2 O
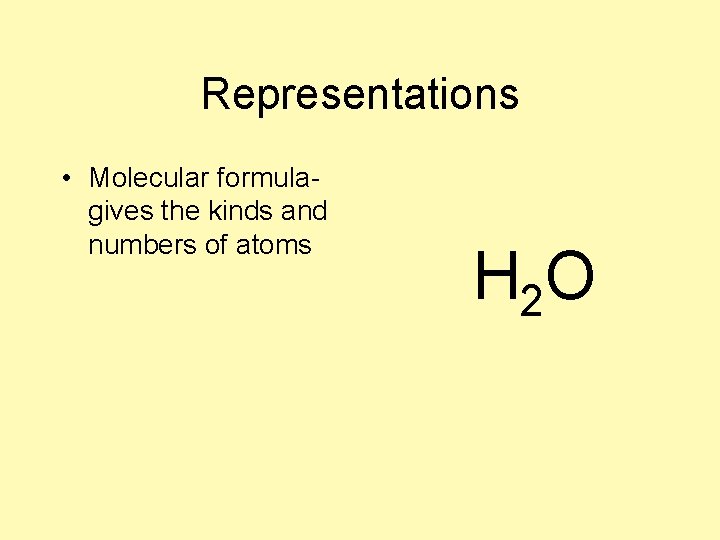
Representations • Molecular formulagives the kinds and numbers of atoms H 2 O
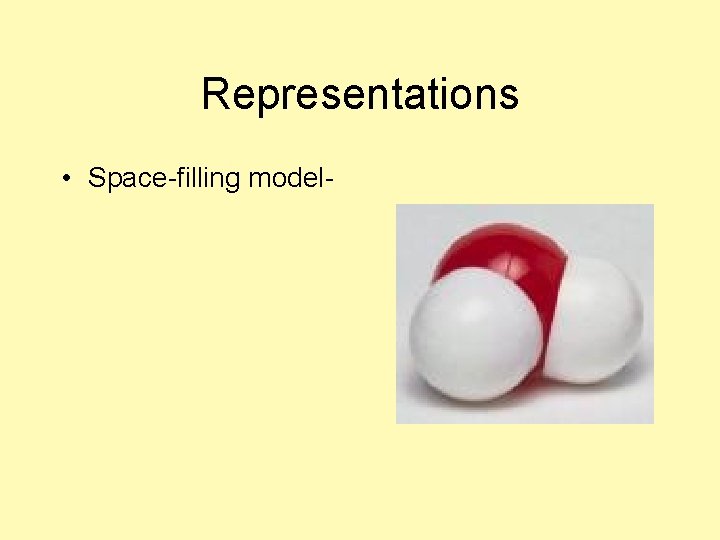
Representations • Space-filling model-
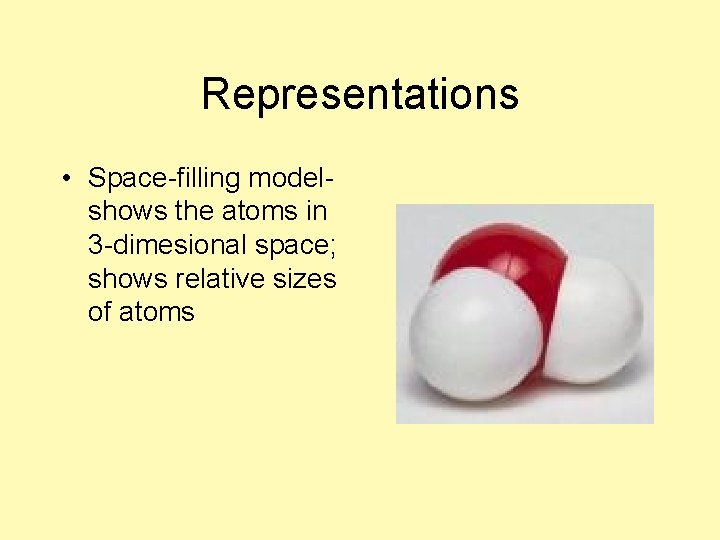
Representations • Space-filling modelshows the atoms in 3 -dimesional space; shows relative sizes of atoms
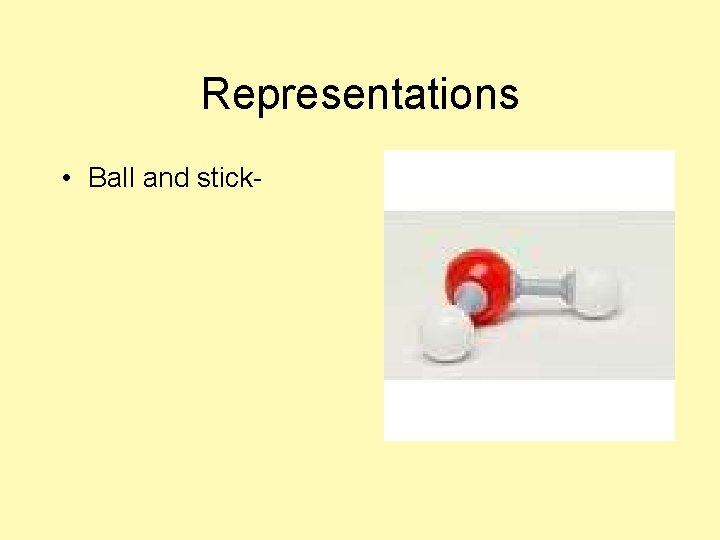
Representations • Ball and stick-
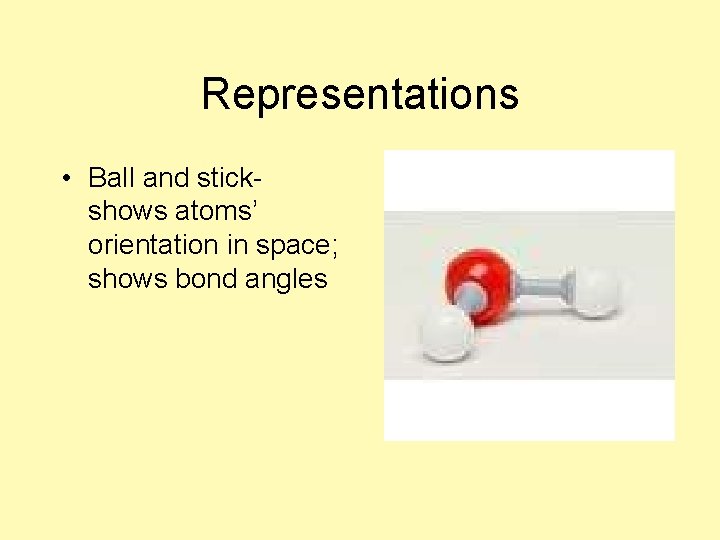
Representations • Ball and stickshows atoms’ orientation in space; shows bond angles
Representations • Perspective drawing -
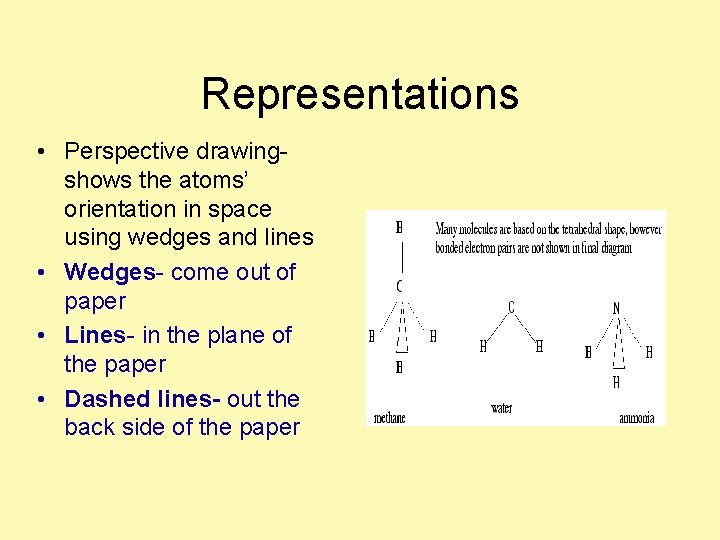
Representations • Perspective drawingshows the atoms’ orientation in space using wedges and lines • Wedges- come out of paper • Lines- in the plane of the paper • Dashed lines- out the back side of the paper
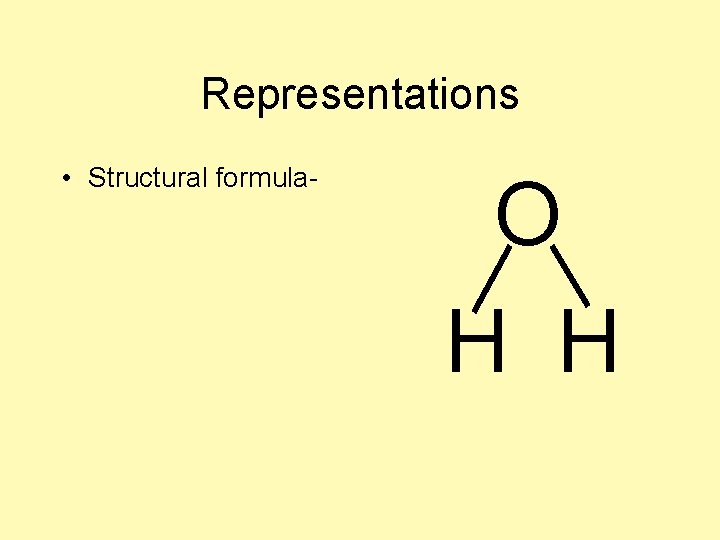
Representations • Structural formula- O H H
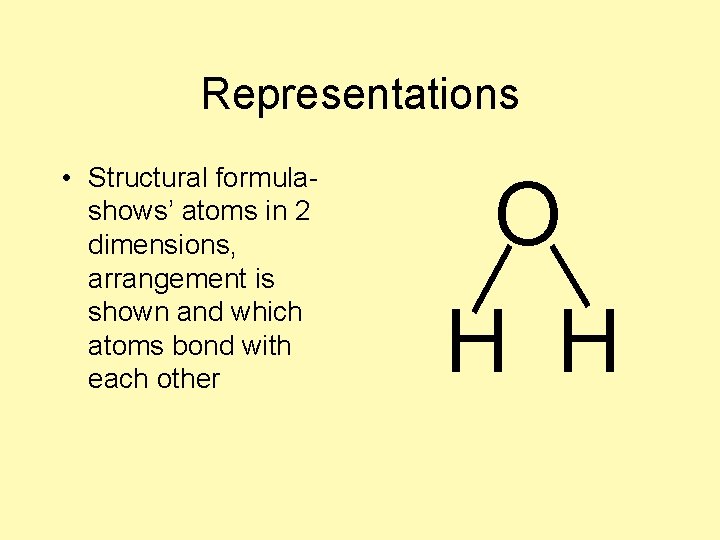
Representations • Structural formulashows’ atoms in 2 dimensions, arrangement is shown and which atoms bond with each other O H H
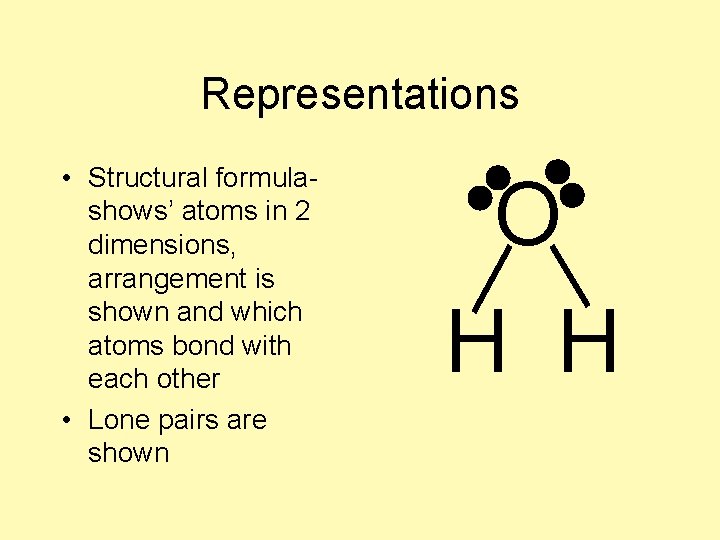
Representations • Structural formulashows’ atoms in 2 dimensions, arrangement is shown and which atoms bond with each other • Lone pairs are shown O H H
- Slides: 26