Molecular Compounds and the Nature of Covalent Bonding
Molecular Compounds and the Nature of Covalent Bonding Chapter 8 Section 1 and 2

Covalent Bonds • Covalent bonds are atoms held together by sharing electrons • These are very different from ionic compounds • They do not give up electrons or accept electrons • A molecule is a neutral group of atoms joined together by covalent bonds.
Molecules • Some elements found in nature form molecules • A molecule is a neutral group of atoms joined together by covalent bonds. • Air contains oxygen molecules. • Each oxygen consists of 2 oxygen joined by covalent bonds • A diatomic molecule is a molecule consisting of two atoms. • An oxygen molecule is a diatomic molecule.
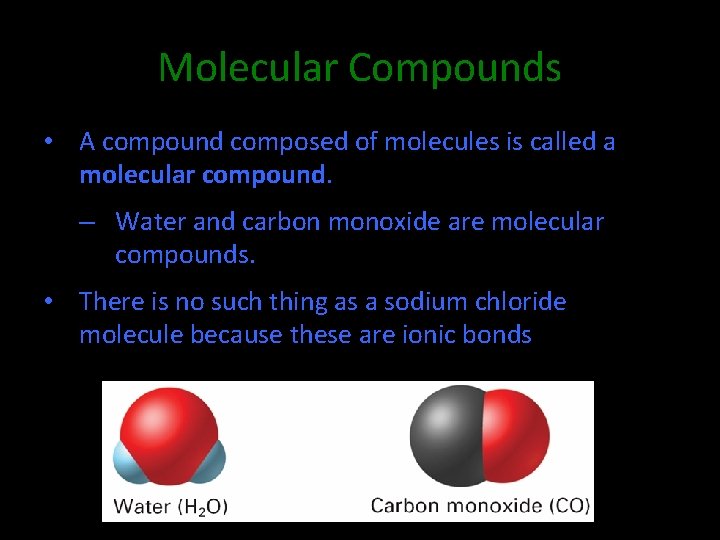
Molecular Compounds • A compound composed of molecules is called a molecular compound. – Water and carbon monoxide are molecular compounds. • There is no such thing as a sodium chloride molecule because these are ionic bonds

Differences of Ionic and covalent Compounds • Molecular compounds tend to have relatively lower melting and boiling points than ionic compounds. • Many molecular compounds are gases or liquids at room temperature

Differences of Ionic and covalent Compounds • Ionic compounds are formed from metals and nonmetals • Molecular compounds are formed from 2 or more nonmetals
Molecular Formula • A molecular formula is the chemical formula of a molecular compound. • A molecular formula shows how many atoms of each element a molecule contains. • The molecular formula for water is H 2 O – 2 hydrogen and 1 water
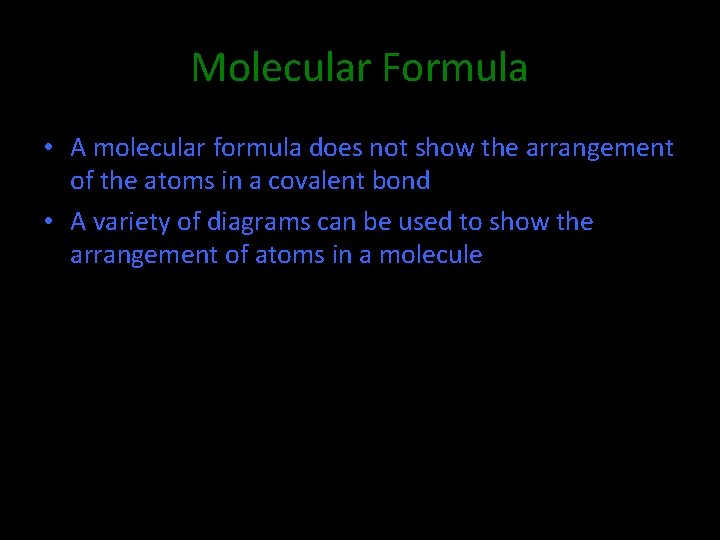
Molecular Formula • A molecular formula does not show the arrangement of the atoms in a covalent bond • A variety of diagrams can be used to show the arrangement of atoms in a molecule

The Octet Rule • Remember, when ionic bonds form electrons are transferred so that each ion achieves a noble gas configuration • In covalent bonds, electron sharing usually occurs so that atoms attain the electron configurations of noble gases.

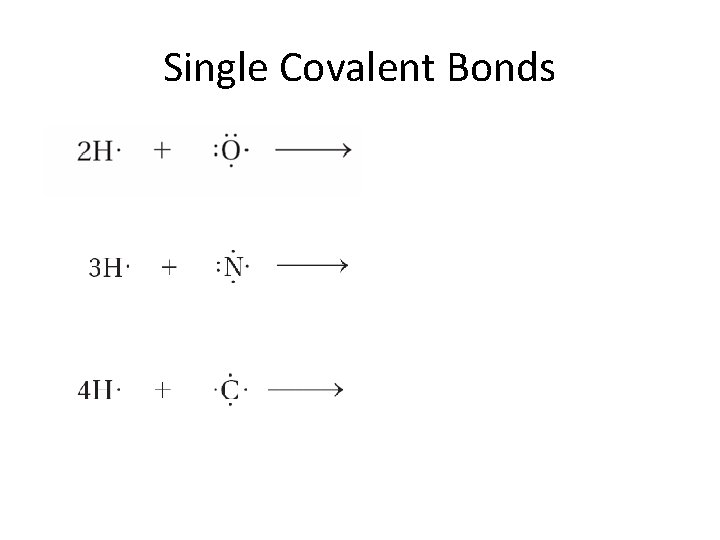
8. 2 The Octet Rule • Each hydrogen atom has one electron. • A pair of hydrogen atoms will share these 2 electrons so that they will both achieve the electron configuration of helium
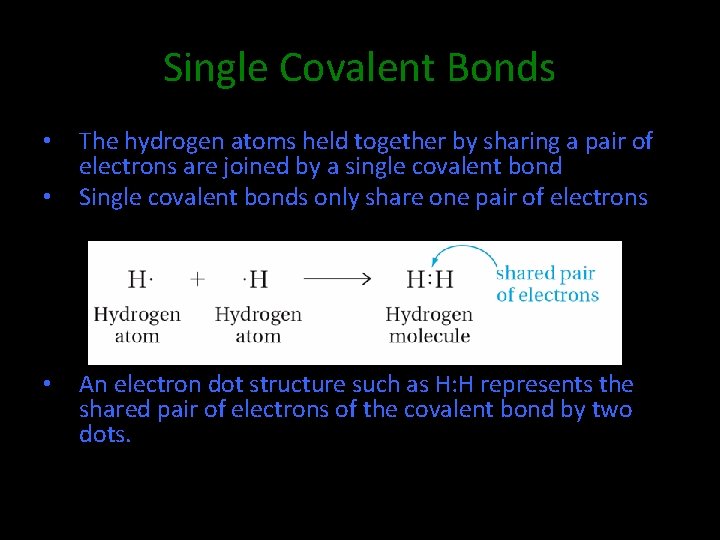
Single Covalent Bonds • • • The hydrogen atoms held together by sharing a pair of electrons are joined by a single covalent bond Single covalent bonds only share one pair of electrons An electron dot structure such as H: H represents the shared pair of electrons of the covalent bond by two dots.
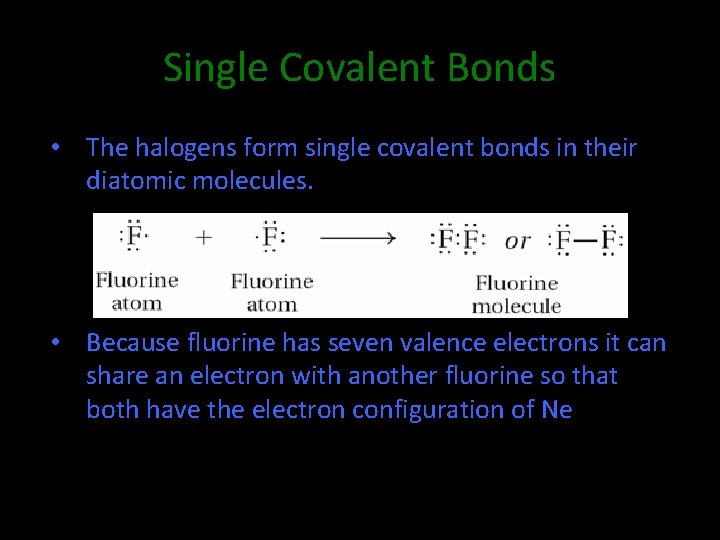
8. 2 Single Covalent Bonds • The halogens form single covalent bonds in their diatomic molecules. • Because fluorine has seven valence electrons it can share an electron with another fluorine so that both have the electron configuration of Ne
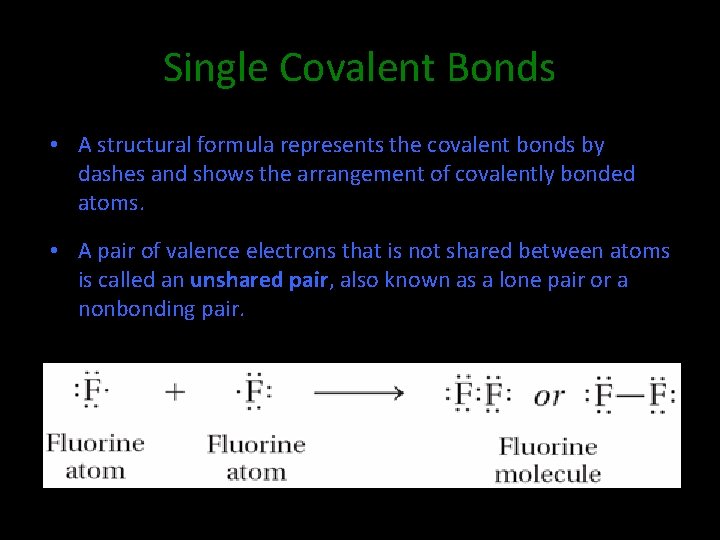
8. 2 Single Covalent Bonds • A structural formula represents the covalent bonds by dashes and shows the arrangement of covalently bonded atoms. • A pair of valence electrons that is not shared between atoms is called an unshared pair, also known as a lone pair or a nonbonding pair.
8. 2 Single Covalent Bonds
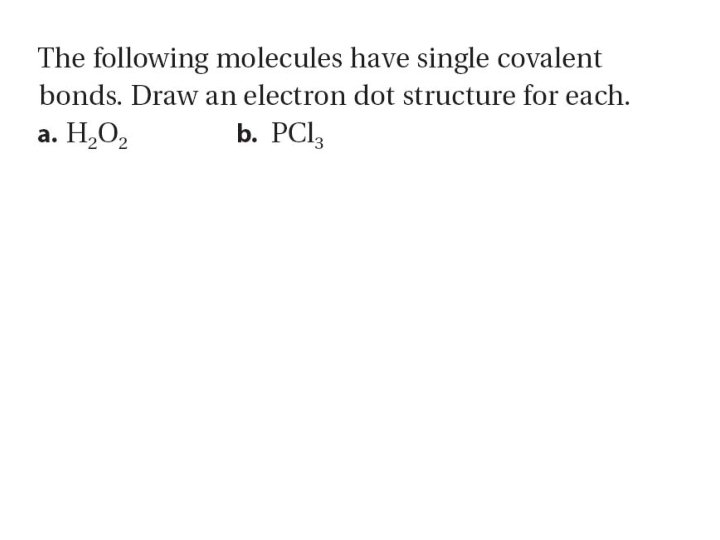

Double and Triple Covalent Bonds – Sometimes atoms bonds by sharing more than one pair of electrons – Atoms form double or triple covalent bonds if they can attain a noble gas structure by sharing two pairs or three pairs of electrons. • A bond that involves two shared pairs of electrons is a double covalent bond. • A bond formed by sharing three pairs of electrons is a triple covalent bond.
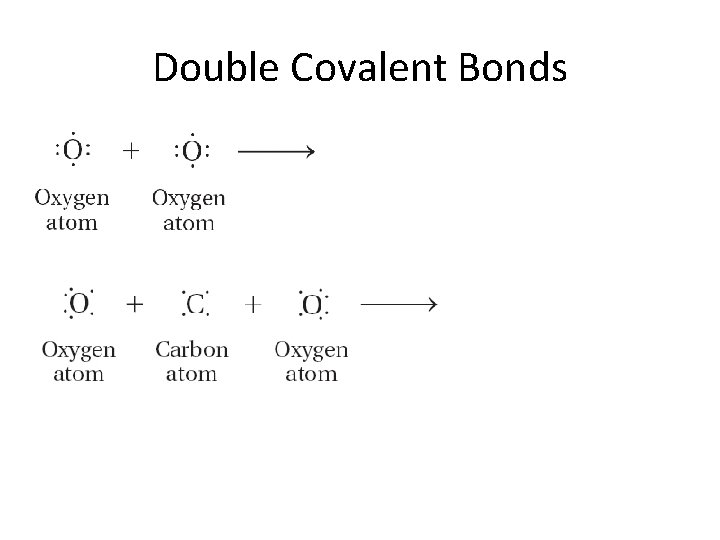
Double Covalent Bonds

Triple Covalent Bonds • Example Nitrogen, N 2
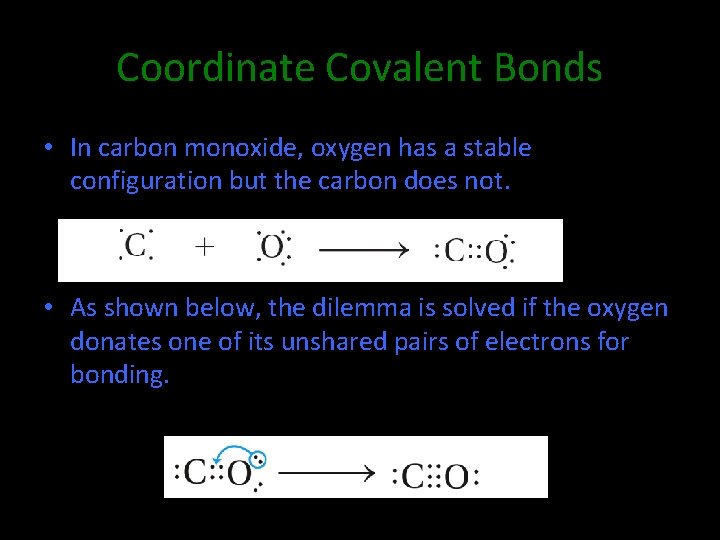
Coordinate Covalent Bonds • In carbon monoxide, oxygen has a stable configuration but the carbon does not. • As shown below, the dilemma is solved if the oxygen donates one of its unshared pairs of electrons for bonding.
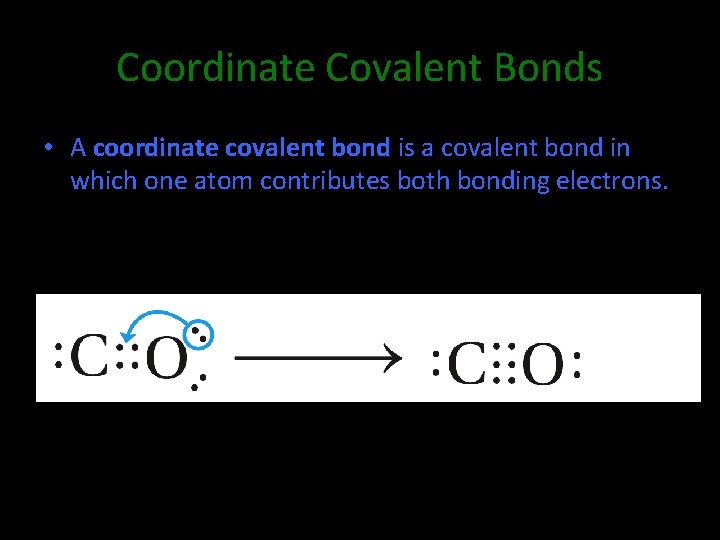
Coordinate Covalent Bonds • A coordinate covalent bond is a covalent bond in which one atom contributes both bonding electrons.
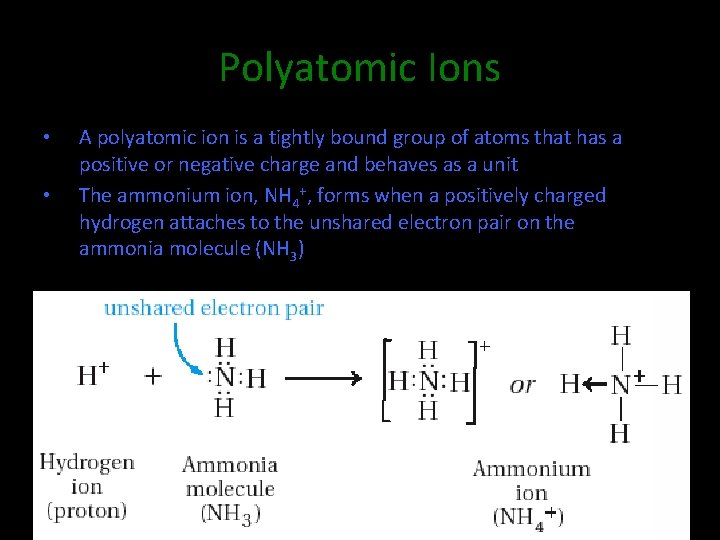
Polyatomic Ions • • A polyatomic ion is a tightly bound group of atoms that has a positive or negative charge and behaves as a unit The ammonium ion, NH 4+, forms when a positively charged hydrogen attaches to the unshared electron pair on the ammonia molecule (NH 3)

Polyatomic Ions • Most polyatomic cation and anions contain both covalent and coordinate covalent bonds • Polyatomic ions contain ionic and covalent bonding
Example • SO 32 -

Bond Dissociation Energy • The energy required to break the bond between two covalently bonded atoms is known as the bond dissociation energy. • A large bond dissociation energy corresponds to a strong covalent bond
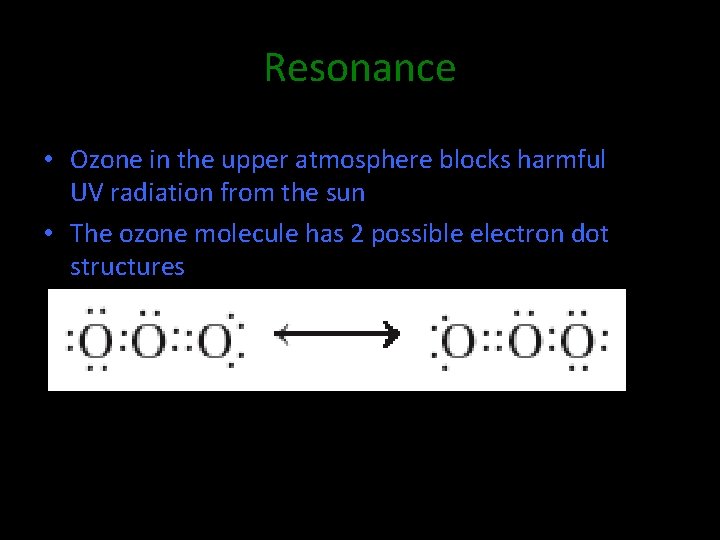
Resonance • Ozone in the upper atmosphere blocks harmful UV radiation from the sun • The ozone molecule has 2 possible electron dot structures
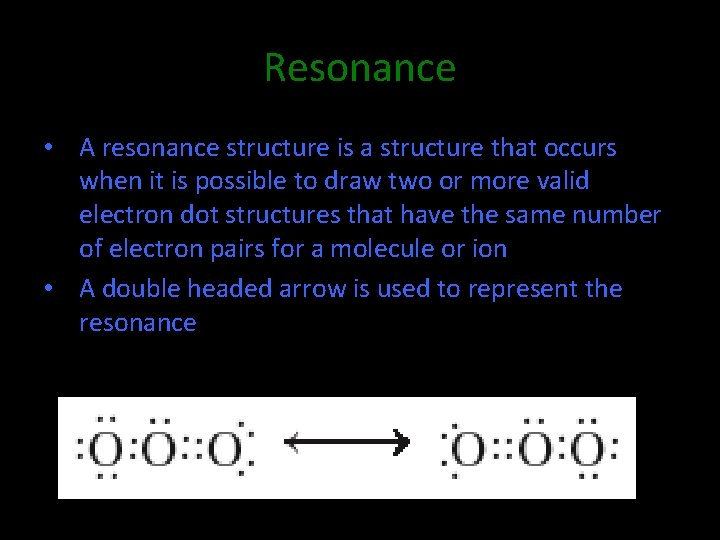
Resonance • A resonance structure is a structure that occurs when it is possible to draw two or more valid electron dot structures that have the same number of electron pairs for a molecule or ion • A double headed arrow is used to represent the resonance

Exceptions to the Octet Rule • Some molecules do not satisfy the octet rule • The octet rule cannot be satisfied in molecules whose total number of valence electrons is an odd number. • Example nitrogen dioxide (NO 2) contains a total of 17 valence electrons

Exceptions to the Octet Rule • BF 3 • PCl 5 • SF 5
- Slides: 30