Molecular Bonding Theories VSEPR Start with Lewis Structure
Molecular Bonding Theories
VSEPR �Start with Lewis Structure – can determine molecular geometry �Each bond separate �Predicts Shapes does NOT explain �Nothing about e- energies
Valence Bond Theory �ANOTHER MODEL OF BONDING! �What it does: �Focus on individual bonds in a molecule �Predicts shapes of molecules - hybridization �Predicts bond order �What it doesn’t: �Predict bond energies �Describe electron distributions in a molecule
Molecular Orbital Theory �Like AO’s but for molecules – compute standing wave patterns appropriate for multiple nuclei and multiple electrons: �Unique set for each kind of molecule �Describes whole molecule, not individual bonds �Describes all molecular properties, including spectra, polarity, charge distribution, magnetism, radicals �Every molecule unique set �Energies and spectra �Hard to use without a computer
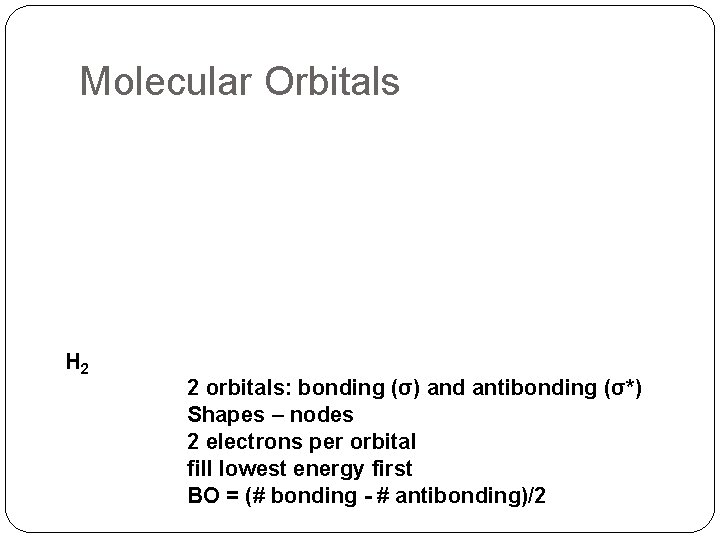
Molecular Orbitals H 2 2 orbitals: bonding (σ) and antibonding (σ*) Shapes – nodes 2 electrons per orbital fill lowest energy first BO = (# bonding - # antibonding)/2
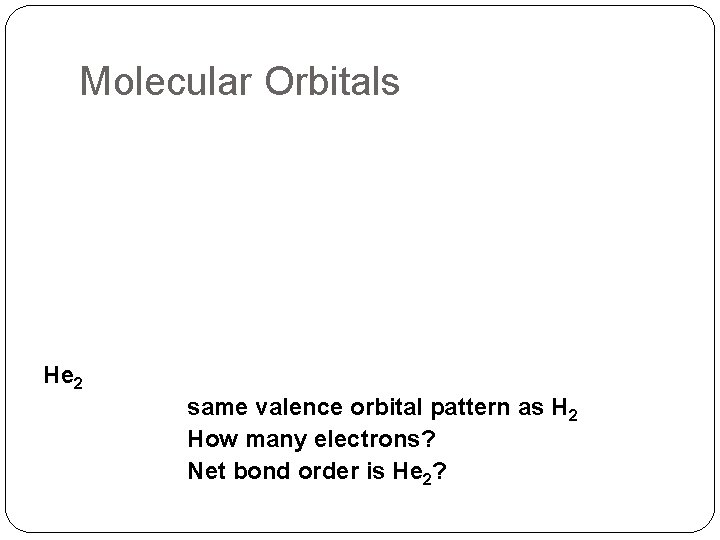
Molecular Orbitals He 2 same valence orbital pattern as H 2 How many electrons? Net bond order is He 2?
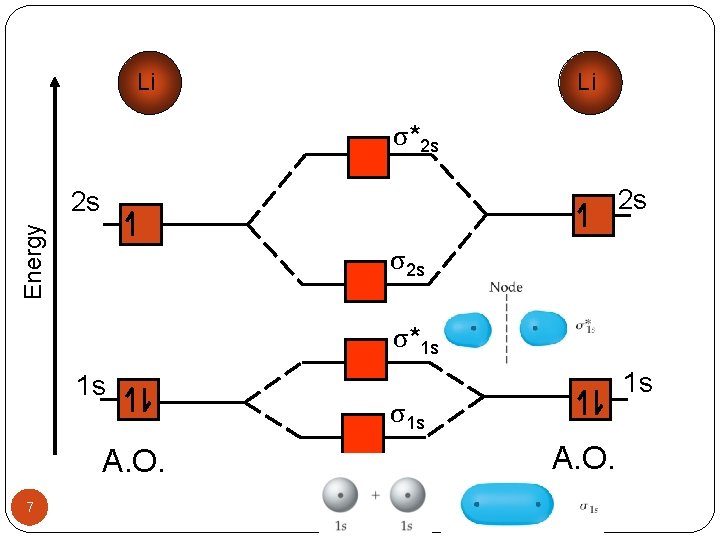
Li Li σ*2 s 2 s Energy 2 s σ*1 s 1 s A. O. 7 1 s σ1 s M. O. A. O.

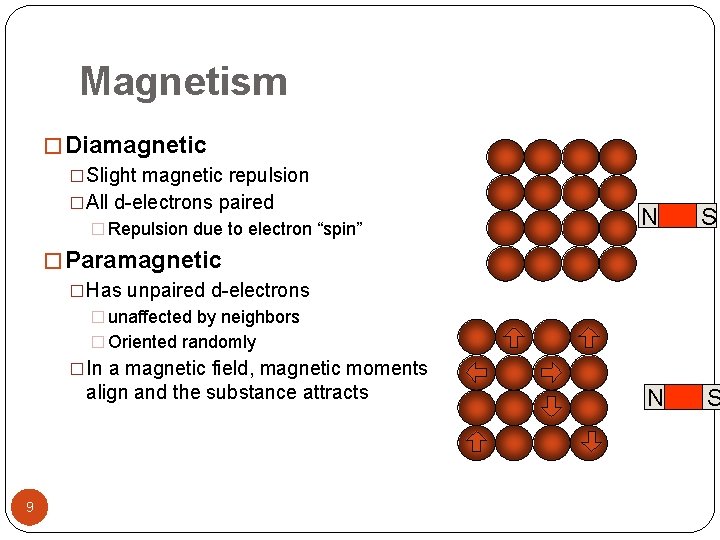
Magnetism � Diamagnetic �Slight magnetic repulsion �All d-electrons paired � Repulsion due to electron “spin” N S � Paramagnetic �Has unpaired d-electrons � unaffected by neighbors � Oriented randomly �In a magnetic field, magnetic moments align and the substance attracts 9
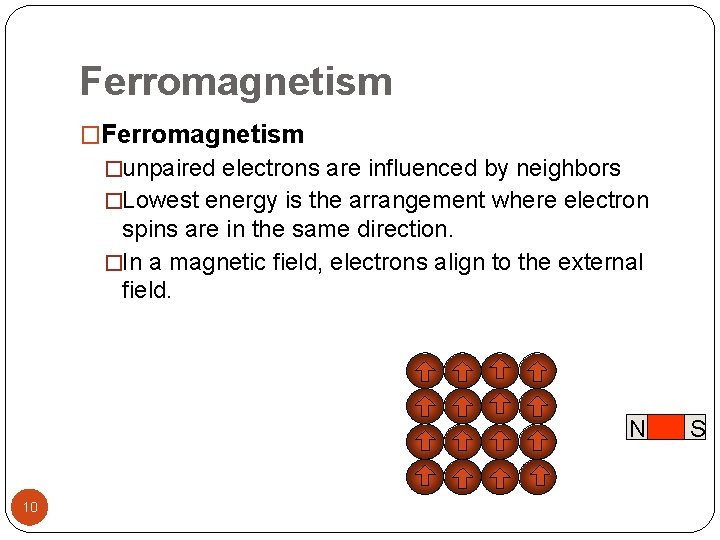
Ferromagnetism �unpaired electrons are influenced by neighbors �Lowest energy is the arrangement where electron spins are in the same direction. �In a magnetic field, electrons align to the external field. N 10 S

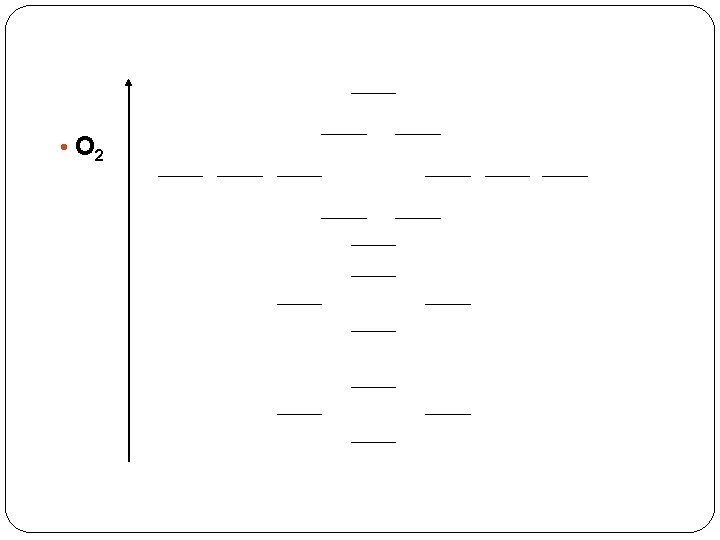
Magnetism of Oxygen? ? 11 Paramagn

Review � LUMO: lowest unoccupied molecular orbital (the first one with out electrons in it) � HOMO: Highest occupied molecular orbital (the highest one that has electrons in it) � SOMO: singly occupied molecular orbital (if it has an unpaired electron) � Paramagnetic: if not all the electrons are paired (this was like the oxygen in the magnet, it stuck on the magnet for a little while) � Diamagnetic: if all the electrons are paired (this was like the nitrogen in the magnet, it just went straight through)
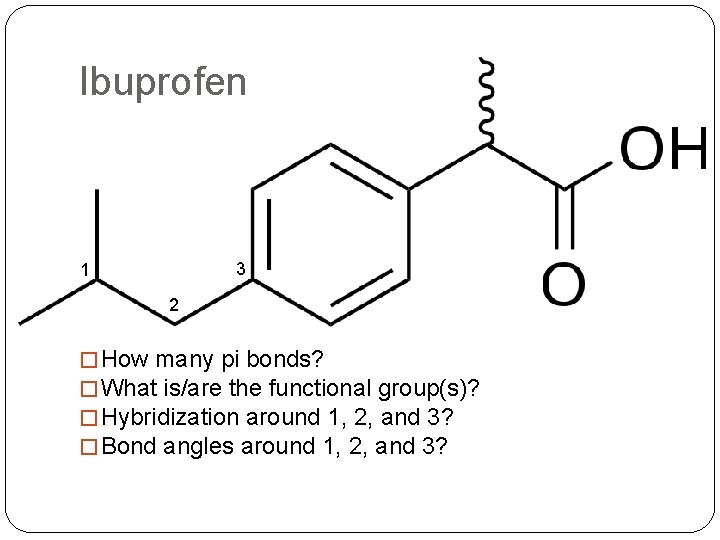
Ibuprofen 3 1 2 � How many pi bonds? � What is/are the functional group(s)? � Hybridization around 1, 2, and 3? � Bond angles around 1, 2, and 3?
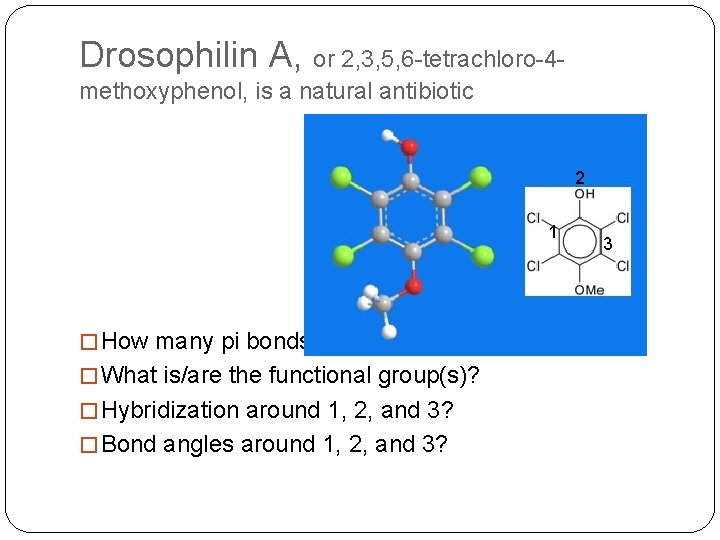
Drosophilin A, or 2, 3, 5, 6 -tetrachloro-4 methoxyphenol, is a natural antibiotic 2 1 � How many pi bonds? � What is/are the functional group(s)? � Hybridization around 1, 2, and 3? � Bond angles around 1, 2, and 3? 3
- Slides: 16