Molecular Biochemistry I Oxidative Phosphorylation Copyright 1999 2007

Molecular Biochemistry I Oxidative Phosphorylation Copyright © 1999 -2007 by Joyce J. Diwan. All rights reserved.
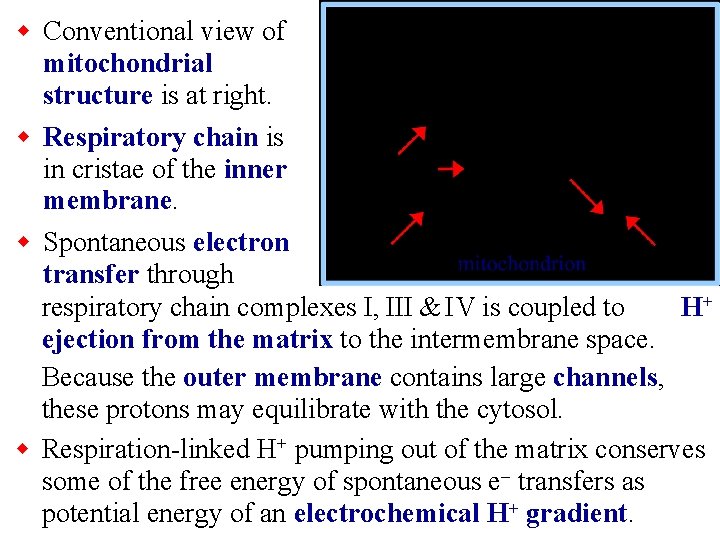
w Conventional view of mitochondrial structure is at right. w Respiratory chain is in cristae of the inner membrane. w Spontaneous electron transfer through respiratory chain complexes I, III & IV is coupled to H+ ejection from the matrix to the intermembrane space. Because the outer membrane contains large channels, these protons may equilibrate with the cytosol. w Respiration-linked H+ pumping out of the matrix conserves some of the free energy of spontaneous e- transfers as potential energy of an electrochemical H+ gradient.
3 -D reconstructions based on electron micrographs of isolated mitochondria taken with a large depth of field, at different tilt angles have indicated that the infoldings of the inner mitochondrial membrane are variable in shape and are connected to the periphery and to each other by narrow tubular regions.
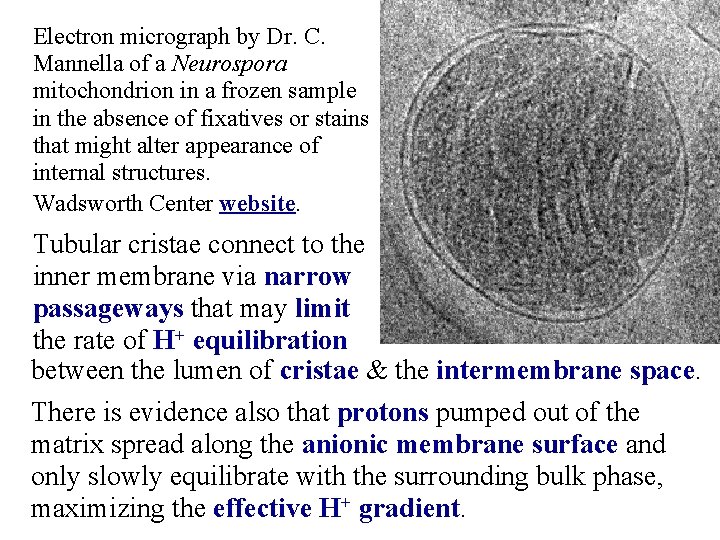
Electron micrograph by Dr. C. Mannella of a Neurospora mitochondrion in a frozen sample in the absence of fixatives or stains that might alter appearance of internal structures. Wadsworth Center website. Tubular cristae connect to the inner membrane via narrow passageways that may limit the rate of H+ equilibration between the lumen of cristae & the intermembrane space. There is evidence also that protons pumped out of the matrix spread along the anionic membrane surface and only slowly equilibrate with the surrounding bulk phase, maximizing the effective H+ gradient.
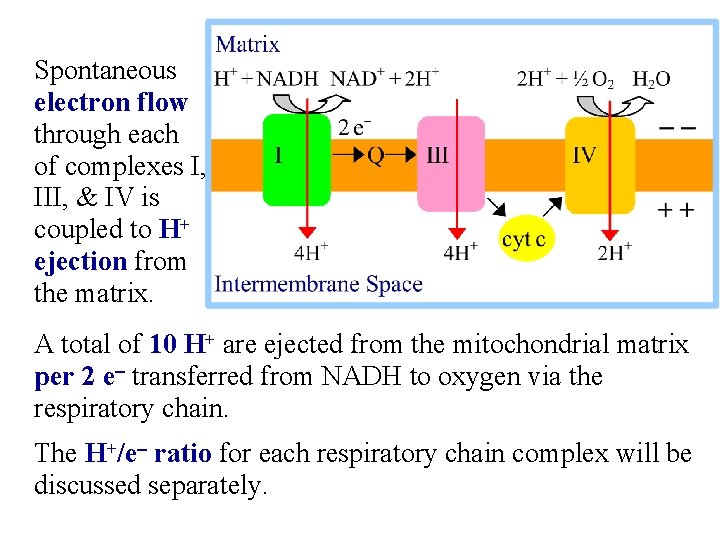
Spontaneous electron flow through each of complexes I, III, & IV is coupled to H+ ejection from the matrix. A total of 10 H+ are ejected from the mitochondrial matrix per 2 e- transferred from NADH to oxygen via the respiratory chain. The H+/e- ratio for each respiratory chain complex will be discussed separately.
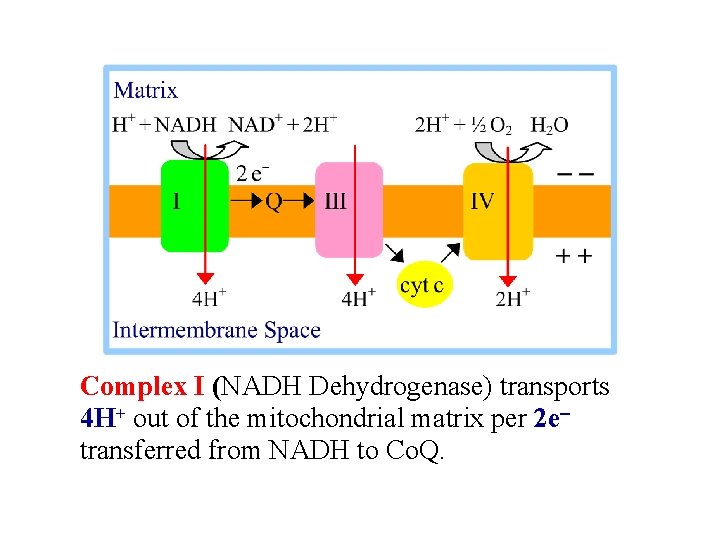
Complex I (NADH Dehydrogenase) transports 4 H+ out of the mitochondrial matrix per 2 e- transferred from NADH to Co. Q.
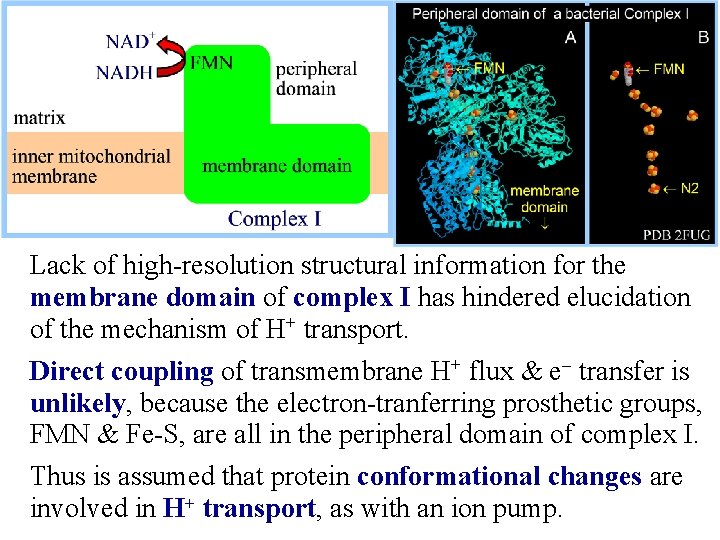
Lack of high-resolution structural information for the membrane domain of complex I has hindered elucidation of the mechanism of H+ transport. Direct coupling of transmembrane H+ flux & e- transfer is unlikely, because the electron-tranferring prosthetic groups, FMN & Fe-S, are all in the peripheral domain of complex I. Thus is assumed that protein conformational changes are involved in H+ transport, as with an ion pump.
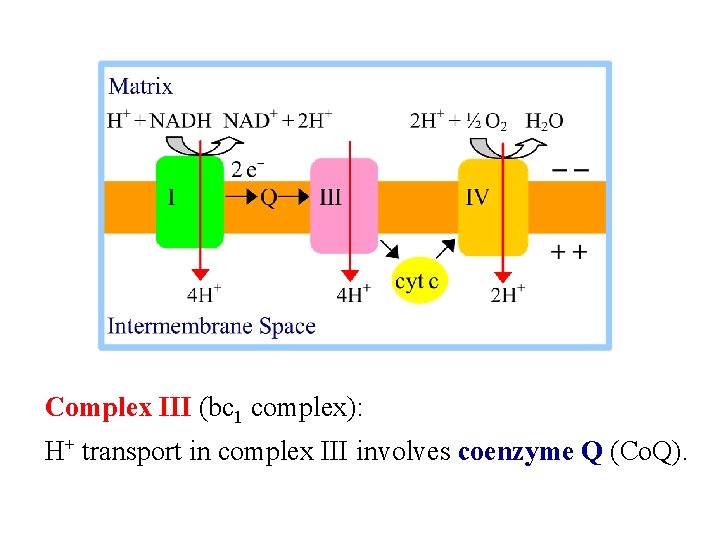
Complex III (bc 1 complex): H+ transport in complex III involves coenzyme Q (Co. Q).
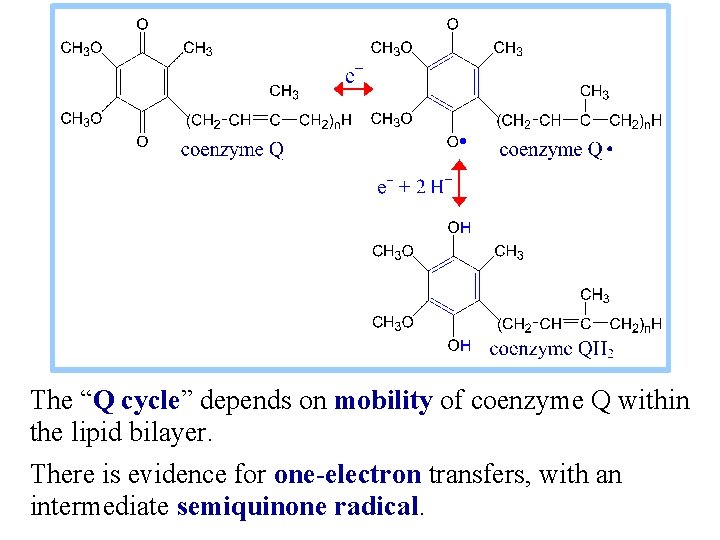
The “Q cycle” depends on mobility of coenzyme Q within the lipid bilayer. There is evidence for one-electron transfers, with an intermediate semiquinone radical.
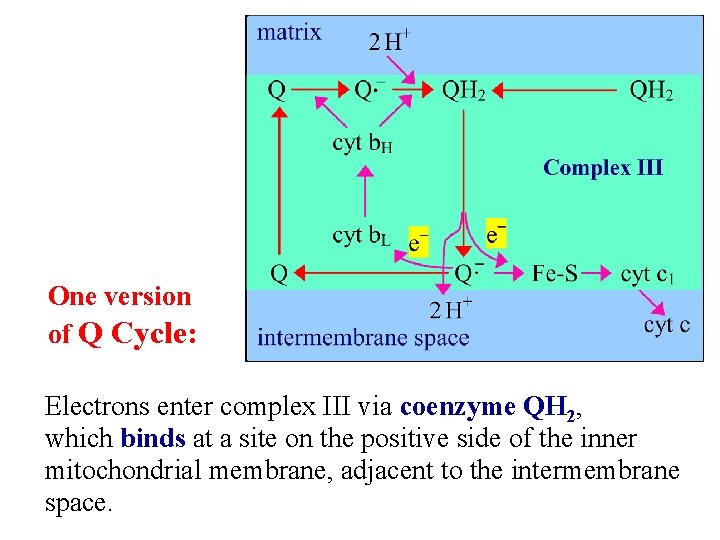
One version of Q Cycle: Electrons enter complex III via coenzyme QH 2, which binds at a site on the positive side of the inner mitochondrial membrane, adjacent to the intermembrane space.
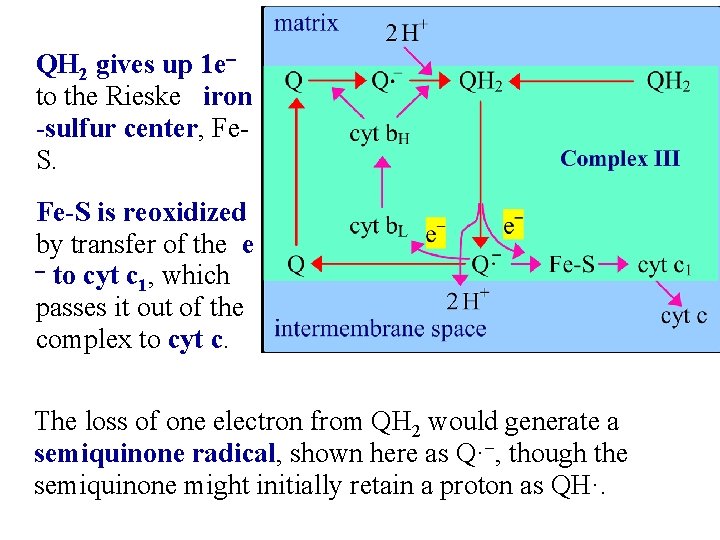
QH 2 gives up 1 eto the Rieske iron -sulfur center, Fe. S. Fe-S is reoxidized by transfer of the e - to cyt c , which 1 passes it out of the complex to cyt c. The loss of one electron from QH 2 would generate a semiquinone radical, shown here as Q·-, though the semiquinone might initially retain a proton as QH·.
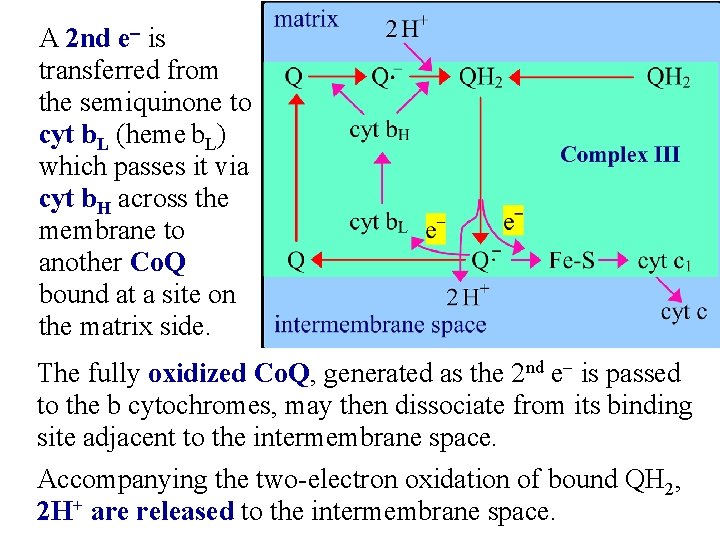
A 2 nd e- is transferred from the semiquinone to cyt b. L (heme b. L) which passes it via cyt b. H across the membrane to another Co. Q bound at a site on the matrix side. The fully oxidized Co. Q, generated as the 2 nd e- is passed to the b cytochromes, may then dissociate from its binding site adjacent to the intermembrane space. Accompanying the two-electron oxidation of bound QH 2, 2 H+ are released to the intermembrane space.
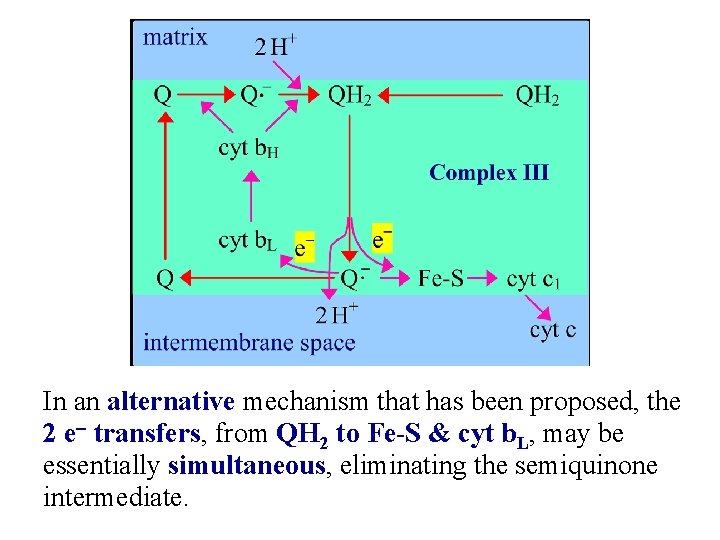
In an alternative mechanism that has been proposed, the 2 e- transfers, from QH 2 to Fe-S & cyt b. L, may be essentially simultaneous, eliminating the semiquinone intermediate.
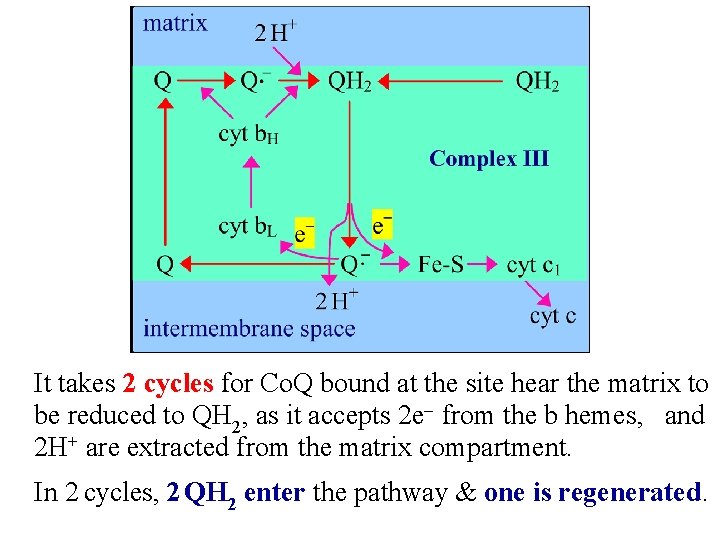
It takes 2 cycles for Co. Q bound at the site hear the matrix to be reduced to QH 2, as it accepts 2 e- from the b hemes, and 2 H+ are extracted from the matrix compartment. In 2 cycles, 2 QH 2 enter the pathway & one is regenerated.
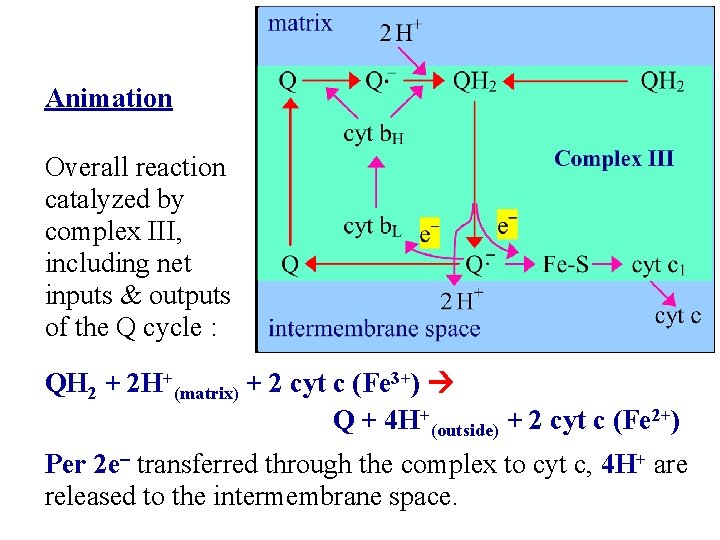
Animation Overall reaction catalyzed by complex III, including net inputs & outputs of the Q cycle : QH 2 + 2 H+(matrix) + 2 cyt c (Fe 3+) Q + 4 H+(outside) + 2 cyt c (Fe 2+) Per 2 e- transferred through the complex to cyt c, 4 H+ are released to the intermembrane space.
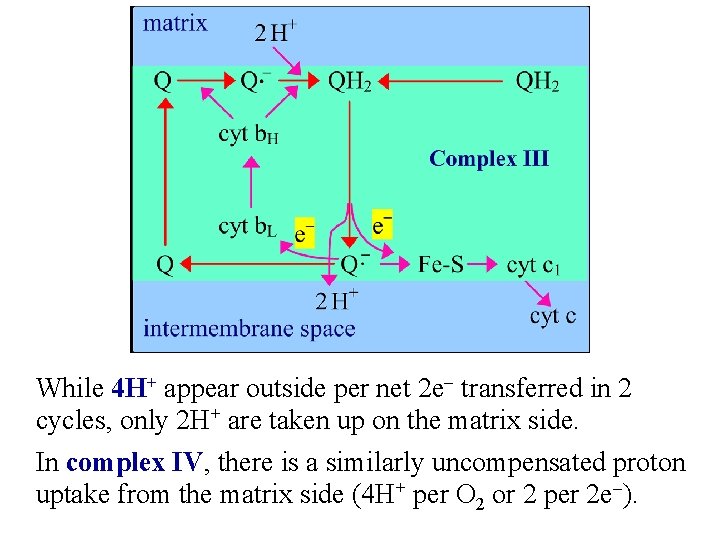
While 4 H+ appear outside per net 2 e- transferred in 2 cycles, only 2 H+ are taken up on the matrix side. In complex IV, there is a similarly uncompensated proton uptake from the matrix side (4 H+ per O 2 or 2 per 2 e-).
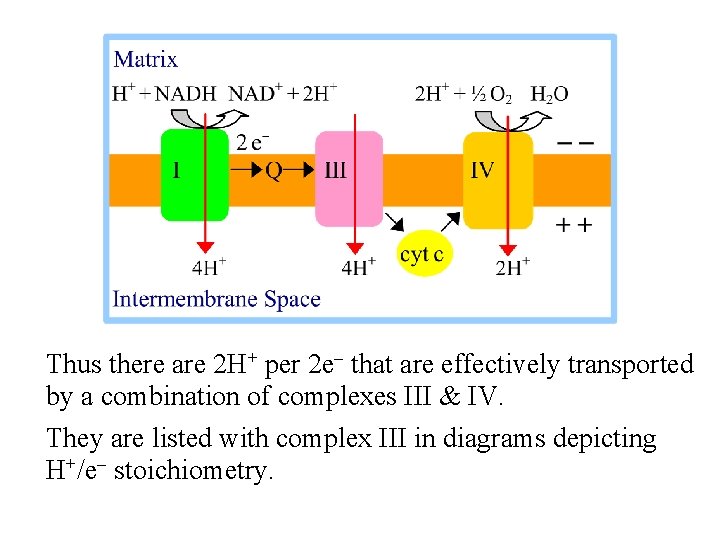
Thus there are 2 H+ per 2 e- that are effectively transported by a combination of complexes III & IV. They are listed with complex III in diagrams depicting H+/e- stoichiometry.
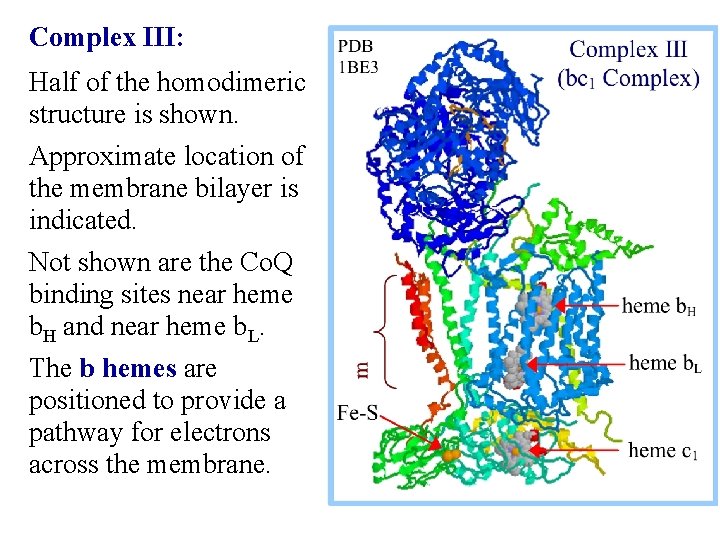
Complex III: Half of the homodimeric structure is shown. Approximate location of the membrane bilayer is indicated. Not shown are the Co. Q binding sites near heme b. H and near heme b. L. The b hemes are positioned to provide a pathway for electrons across the membrane.
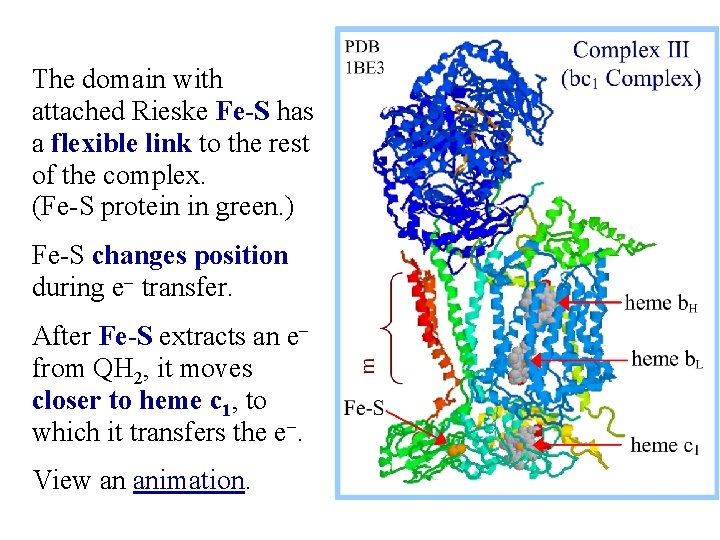
The domain with attached Rieske Fe-S has a flexible link to the rest of the complex. (Fe-S protein in green. ) Fe-S changes position during e- transfer. After Fe-S extracts an efrom QH 2, it moves closer to heme c 1, to which it transfers the e-. View an animation.
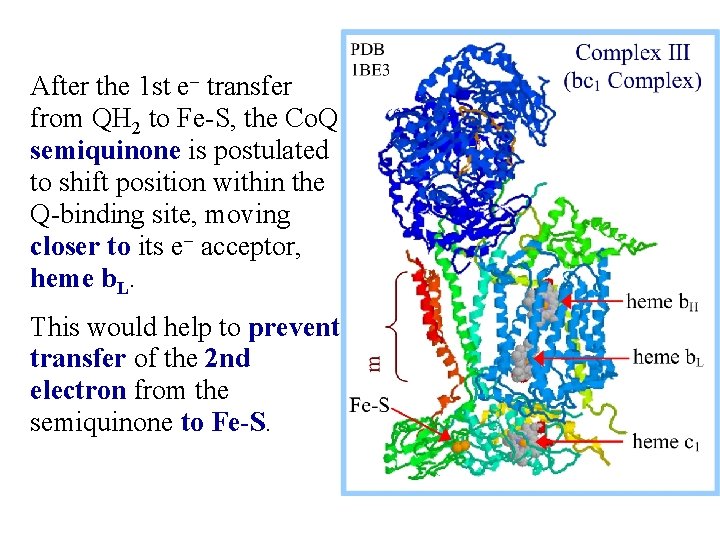
After the 1 st e- transfer from QH 2 to Fe-S, the Co. Q semiquinone is postulated to shift position within the Q-binding site, moving closer to its e- acceptor, heme b. L. This would help to prevent transfer of the 2 nd electron from the semiquinone to Fe-S.
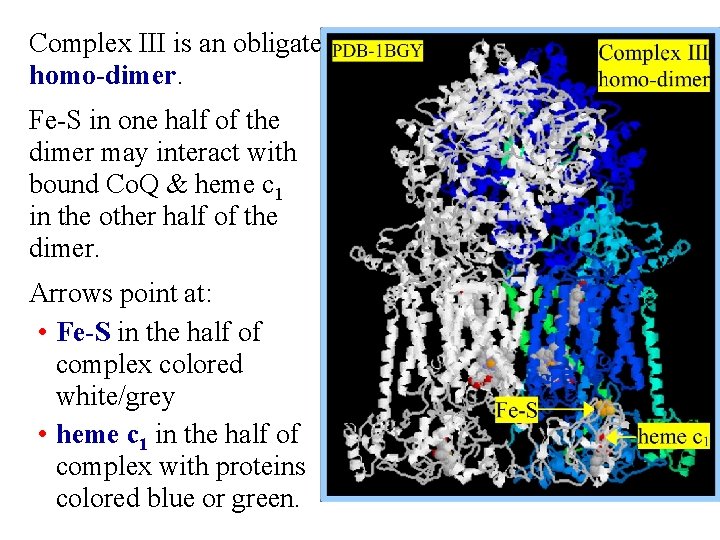
Complex III is an obligate homo-dimer. Fe-S in one half of the dimer may interact with bound Co. Q & heme c 1 in the other half of the dimer. Arrows point at: • Fe-S in the half of complex colored white/grey • heme c 1 in the half of complex with proteins colored blue or green.
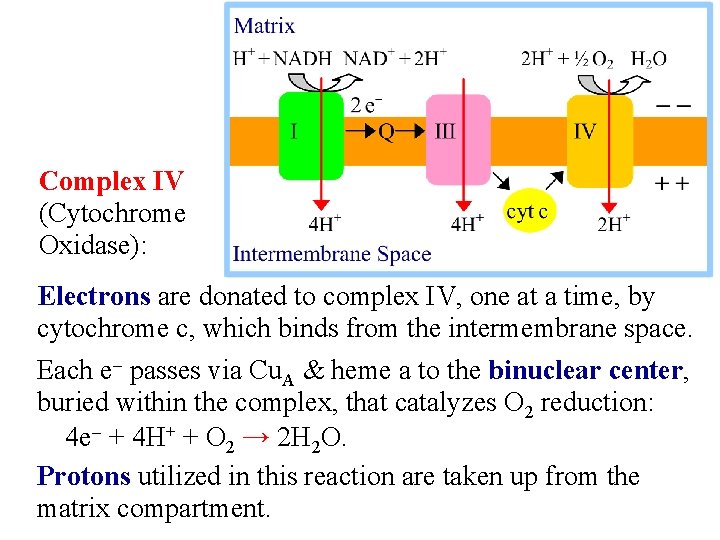
Complex IV (Cytochrome Oxidase): Electrons are donated to complex IV, one at a time, by cytochrome c, which binds from the intermembrane space. Each e- passes via Cu. A & heme a to the binuclear center, buried within the complex, that catalyzes O 2 reduction: 4 e- + 4 H+ + O 2 → 2 H 2 O. Protons utilized in this reaction are taken up from the matrix compartment.
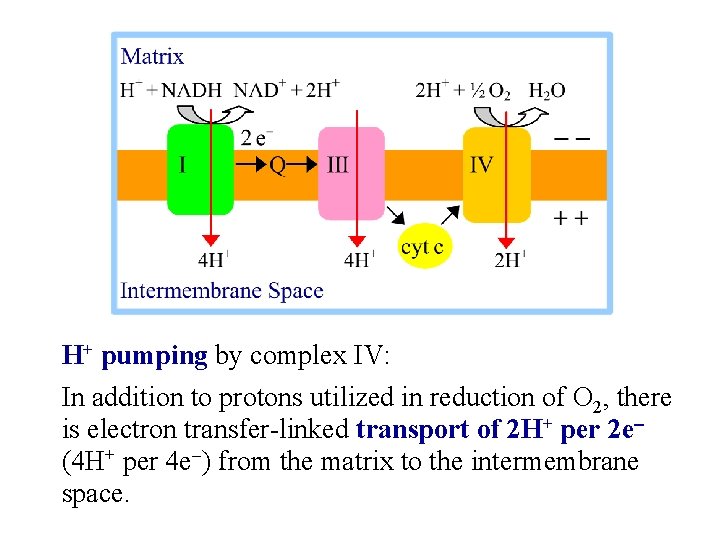
H+ pumping by complex IV: In addition to protons utilized in reduction of O 2, there is electron transfer-linked transport of 2 H+ per 2 e- (4 H+ per 4 e-) from the matrix to the intermembrane space.
Structural & mutational studies indicate that protons pass through complex IV via chains of groups subject to protonation/deprotonation, called "proton wires. " These consist mainly of chains of buried water molecules, along with amino acid side-chains, & propionate sidechains of hemes. Separate H+-conducting pathways link each side of the membrane to the buried binuclear center where O 2 reduction takes place. These include 2 proton pathways, designated "D" & "K" (named after constituent Asp & Lys residues) extending from the mitochondrial matrix to near the binuclear center deep within complex IV. Images in web pages of: IBI, & Crofts.
A switch mechanism controlled by the reaction cycle is proposed to effect transfer of a proton from one half-wire (half-channel) to the other. There cannot be an open pathway for H+ completely through the membrane, or oxidative phosphorylation would be uncoupled. (Pumped protons would leak back. ) Switching may involve conformational changes, and oxidation/reduction-linked changes in p. Ka of groups associated with the catalytic metal centers. Detailed mechanisms have been proposed.
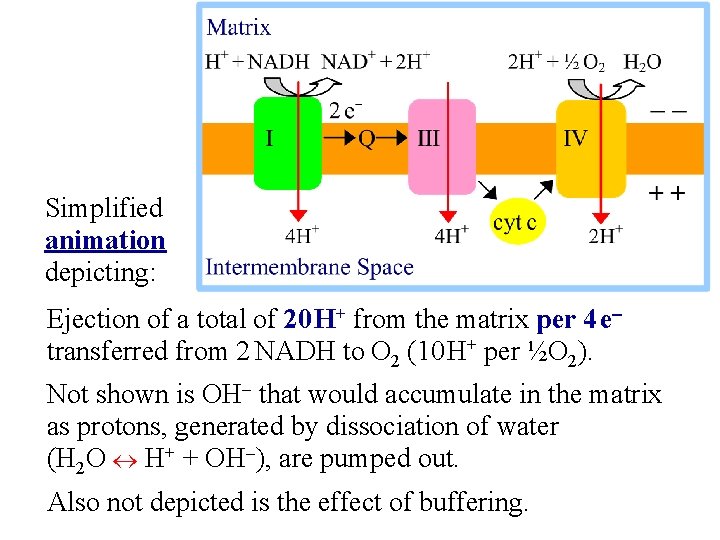
Simplified animation depicting: Ejection of a total of 20 H+ from the matrix per 4 e- transferred from 2 NADH to O 2 (10 H+ per ½O 2). Not shown is OH- that would accumulate in the matrix as protons, generated by dissociation of water (H 2 O H+ + OH-), are pumped out. Also not depicted is the effect of buffering.
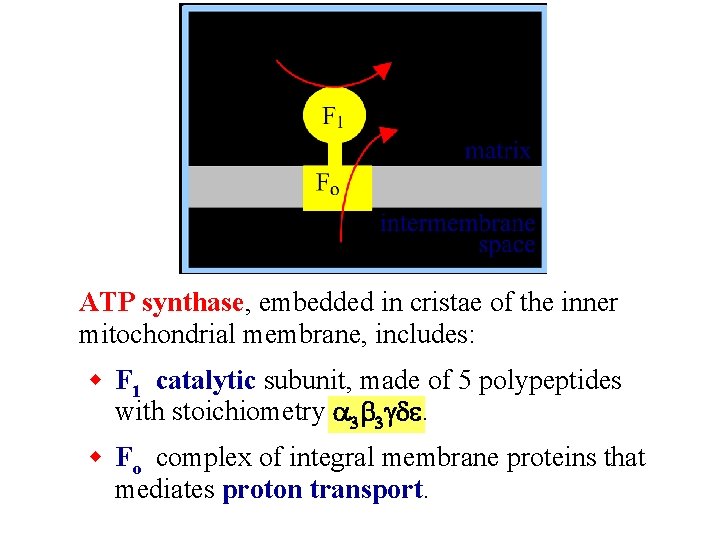
ATP synthase, embedded in cristae of the inner mitochondrial membrane, includes: w F 1 catalytic subunit, made of 5 polypeptides with stoichiometry a 3 b 3 gde. w Fo complex of integral membrane proteins that mediates proton transport.
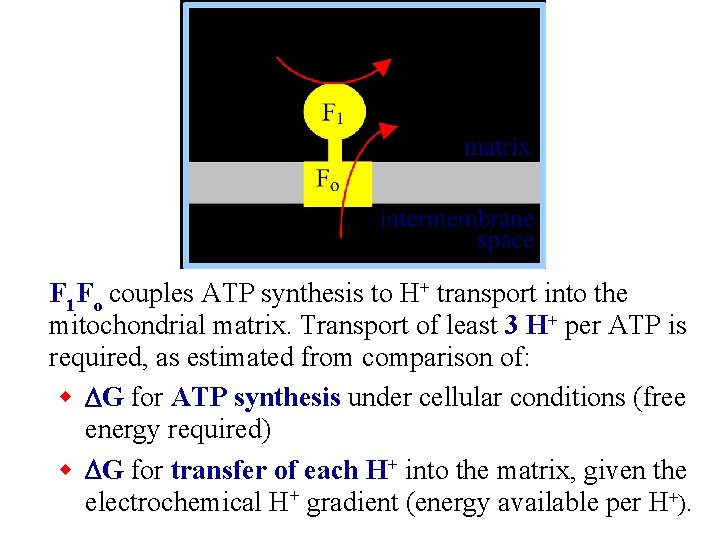
F 1 Fo couples ATP synthesis to H+ transport into the mitochondrial matrix. Transport of least 3 H+ per ATP is required, as estimated from comparison of: w DG for ATP synthesis under cellular conditions (free energy required) w DG for transfer of each H+ into the matrix, given the electrochemical H+ gradient (energy available per H+).
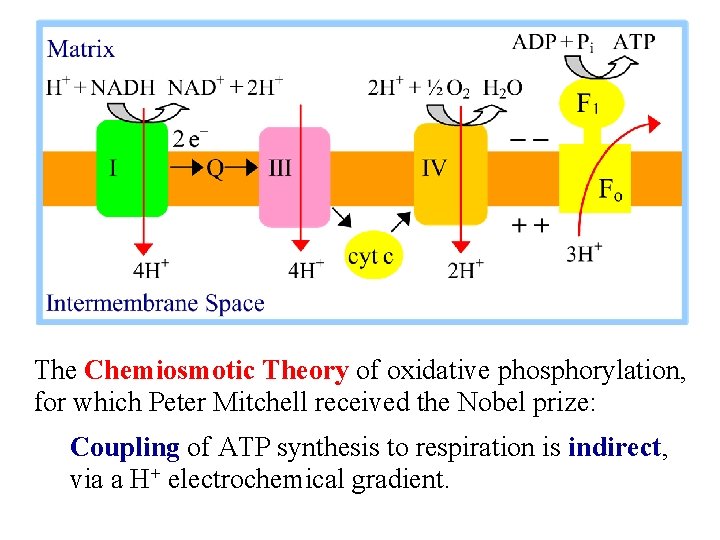
The Chemiosmotic Theory of oxidative phosphorylation, for which Peter Mitchell received the Nobel prize: Coupling of ATP synthesis to respiration is indirect, via a H+ electrochemical gradient.
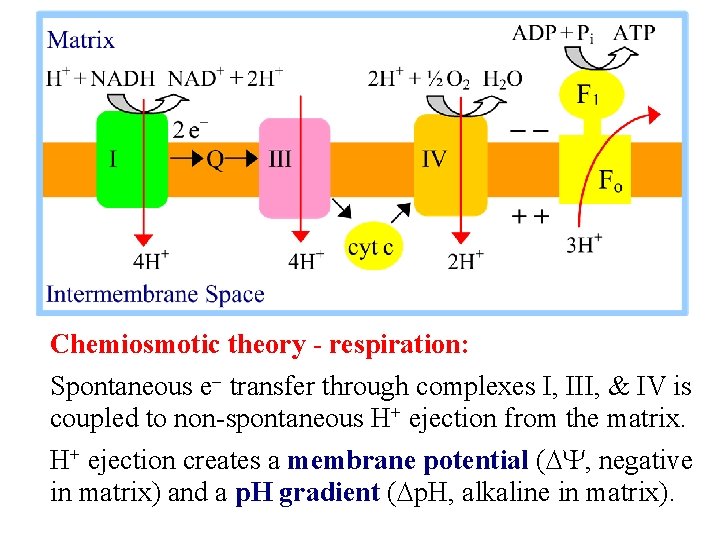
Chemiosmotic theory - respiration: Spontaneous e- transfer through complexes I, III, & IV is coupled to non-spontaneous H+ ejection from the matrix. H+ ejection creates a membrane potential (DY, negative in matrix) and a p. H gradient (Dp. H, alkaline in matrix).
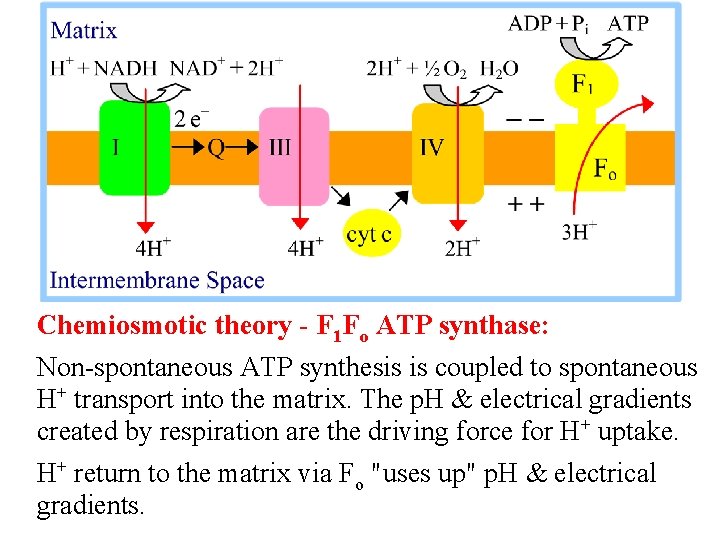
Chemiosmotic theory - F 1 Fo ATP synthase: Non-spontaneous ATP synthesis is coupled to spontaneous H+ transport into the matrix. The p. H & electrical gradients created by respiration are the driving force for H+ uptake. H+ return to the matrix via Fo "uses up" p. H & electrical gradients.
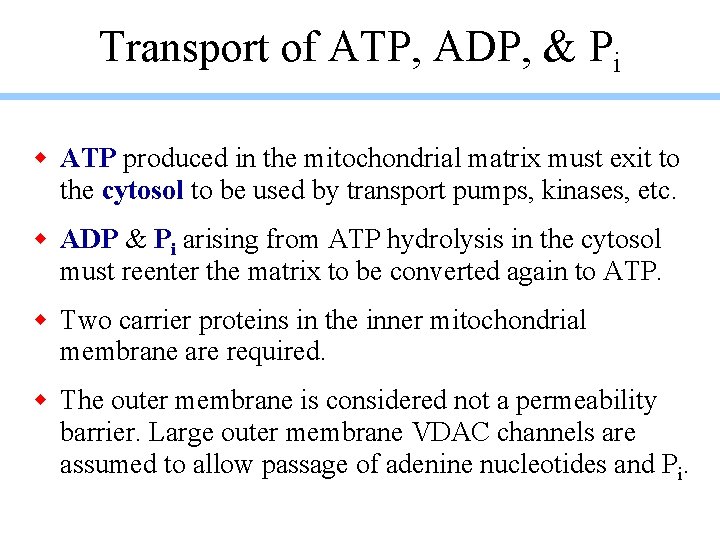
Transport of ATP, ADP, & Pi w ATP produced in the mitochondrial matrix must exit to the cytosol to be used by transport pumps, kinases, etc. w ADP & Pi arising from ATP hydrolysis in the cytosol must reenter the matrix to be converted again to ATP. w Two carrier proteins in the inner mitochondrial membrane are required. w The outer membrane is considered not a permeability barrier. Large outer membrane VDAC channels are assumed to allow passage of adenine nucleotides and Pi.
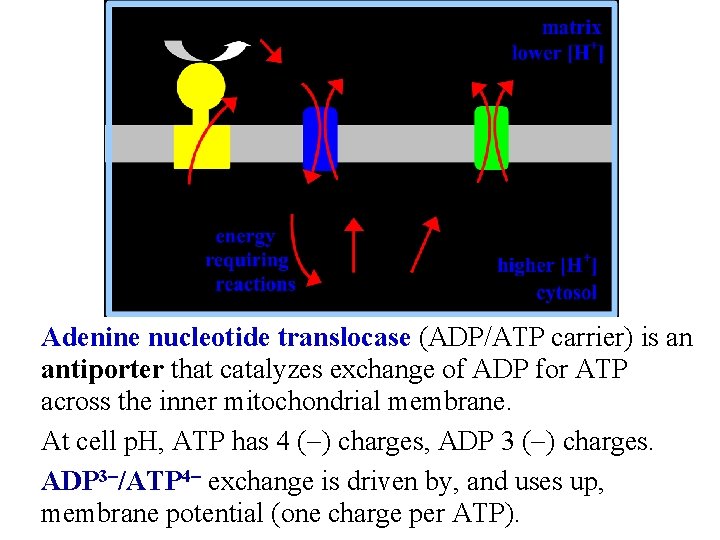
Adenine nucleotide translocase (ADP/ATP carrier) is an antiporter that catalyzes exchange of ADP for ATP across the inner mitochondrial membrane. At cell p. H, ATP has 4 (-) charges, ADP 3 (-) charges. ADP 3 -/ATP 4 - exchange is driven by, and uses up, membrane potential (one charge per ATP).
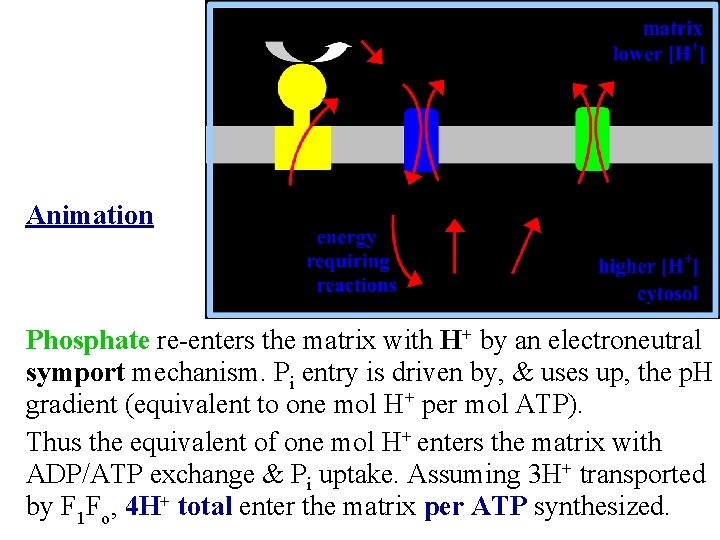
Animation Phosphate re-enters the matrix with H+ by an electroneutral symport mechanism. Pi entry is driven by, & uses up, the p. H gradient (equivalent to one mol H+ per mol ATP). Thus the equivalent of one mol H+ enters the matrix with ADP/ATP exchange & Pi uptake. Assuming 3 H+ transported by F 1 Fo, 4 H+ total enter the matrix per ATP synthesized.
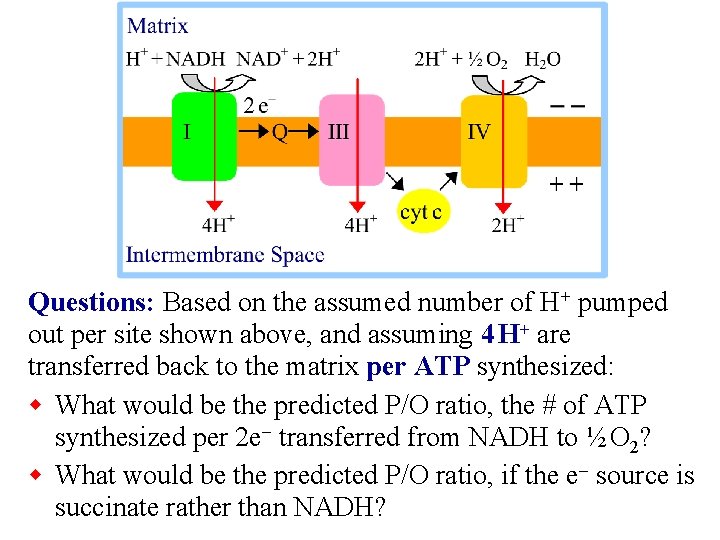
Questions: Based on the assumed number of H+ pumped out per site shown above, and assuming 4 H+ are transferred back to the matrix per ATP synthesized: w What would be the predicted P/O ratio, the # of ATP synthesized per 2 e- transferred from NADH to ½ O 2? w What would be the predicted P/O ratio, if the e- source is succinate rather than NADH?
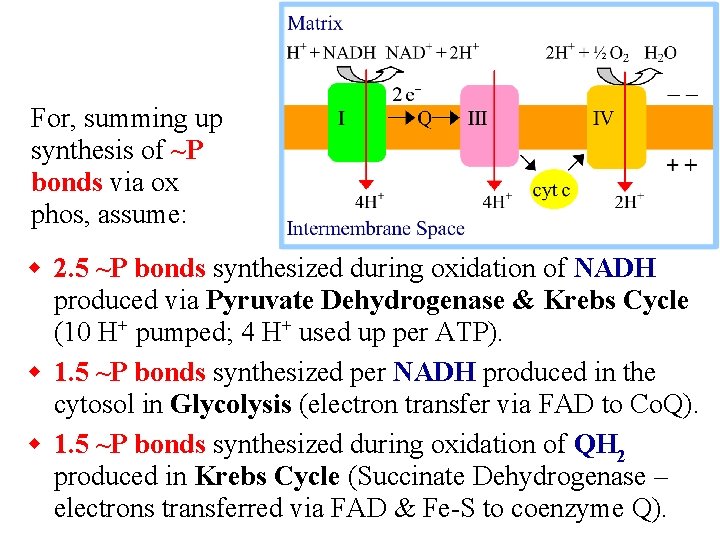
For, summing up synthesis of ~P bonds via ox phos, assume: w 2. 5 ~P bonds synthesized during oxidation of NADH produced via Pyruvate Dehydrogenase & Krebs Cycle (10 H+ pumped; 4 H+ used up per ATP). w 1. 5 ~P bonds synthesized per NADH produced in the cytosol in Glycolysis (electron transfer via FAD to Co. Q). w 1. 5 ~P bonds synthesized during oxidation of QH 2 produced in Krebs Cycle (Succinate Dehydrogenase – electrons transferred via FAD & Fe-S to coenzyme Q).
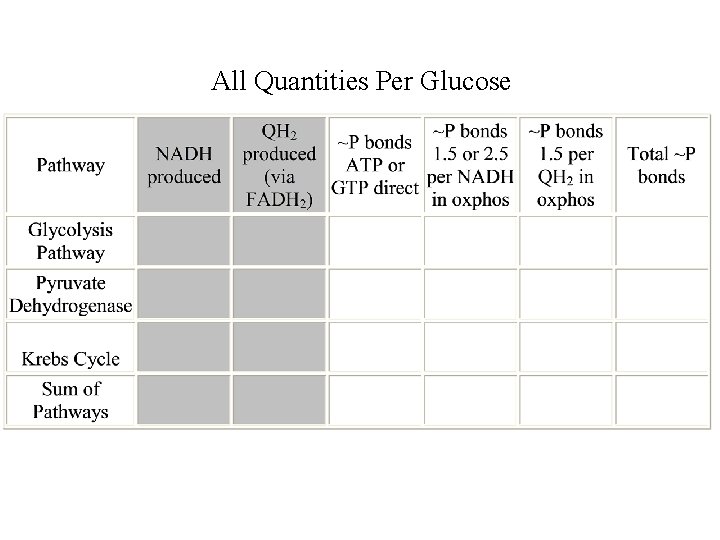
All Quantities Per Glucose
![An oxygen electrode may be used to record [O 2] in a closed vessel. An oxygen electrode may be used to record [O 2] in a closed vessel.](http://slidetodoc.com/presentation_image_h/36cdf8e228e46f143384e0d8585c3762/image-38.jpg)
An oxygen electrode may be used to record [O 2] in a closed vessel. Electron transfer, e. g. , NADH O 2, is monitored by the rate of O 2 disappearance. Above is represented an O 2 electrode recording while mitochondria respire in the presence of Pi and an e- donor (succinate or a substrate of a reaction to generate NADH). The dependence of respiration rate on availability of ADP, the ATP Synthase substrate, is called respiratory control.
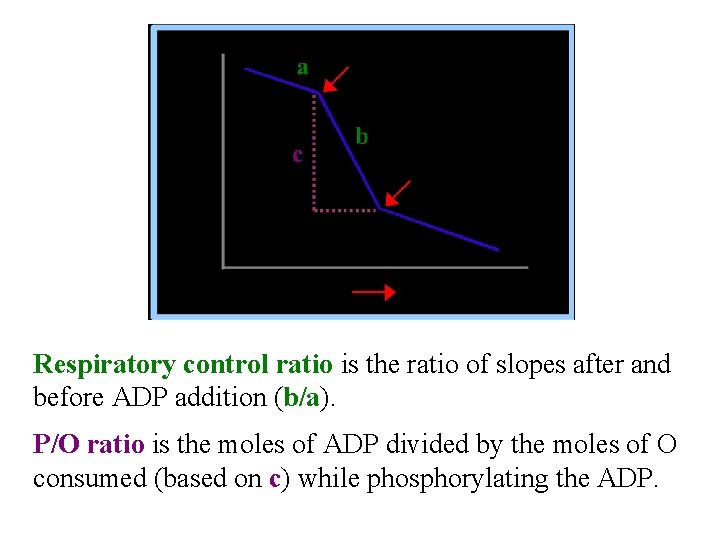
Respiratory control ratio is the ratio of slopes after and before ADP addition (b/a). P/O ratio is the moles of ADP divided by the moles of O consumed (based on c) while phosphorylating the ADP.
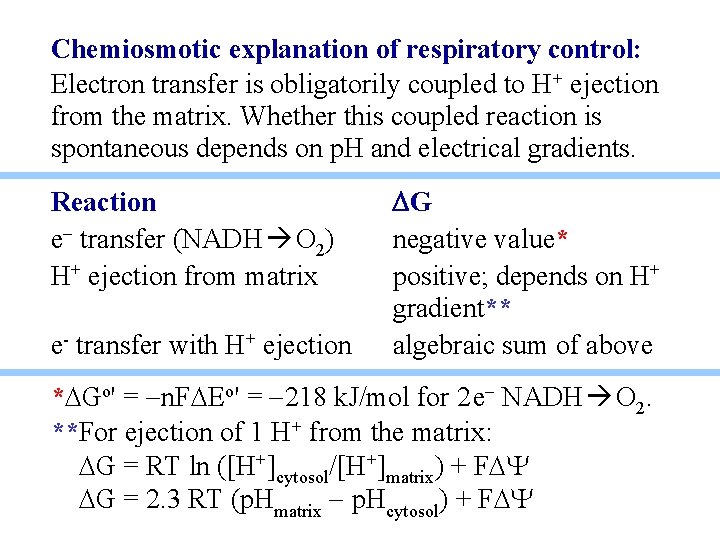
Chemiosmotic explanation of respiratory control: Electron transfer is obligatorily coupled to H+ ejection from the matrix. Whether this coupled reaction is spontaneous depends on p. H and electrical gradients. Reaction e- transfer (NADH O 2) H+ ejection from matrix e- transfer with H+ ejection DG negative value* positive; depends on H+ gradient** algebraic sum of above *DGo' = -n. FDEo' = -218 k. J/mol for 2 e- NADH O 2. **For ejection of 1 H+ from the matrix: DG = RT ln ([H+]cytosol/[H+]matrix) + FDY DG = 2. 3 RT (p. Hmatrix - p. Hcytosol) + FDY
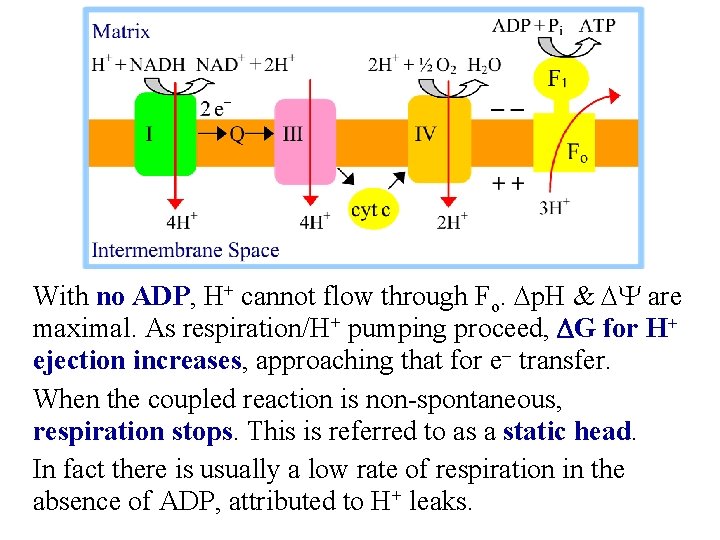
With no ADP, H+ cannot flow through Fo. Dp. H & DY are maximal. As respiration/H+ pumping proceed, DG for H+ ejection increases, approaching that for e- transfer. When the coupled reaction is non-spontaneous, respiration stops. This is referred to as a static head. In fact there is usually a low rate of respiration in the absence of ADP, attributed to H+ leaks.
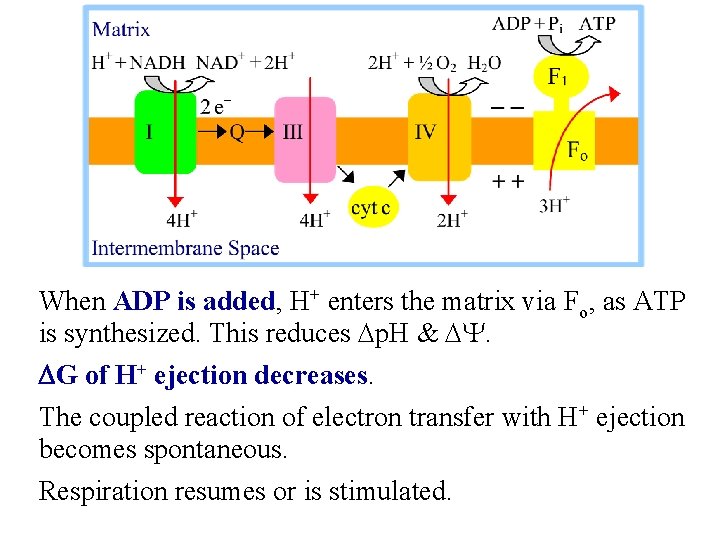
When ADP is added, H+ enters the matrix via Fo, as ATP is synthesized. This reduces Dp. H & DY. DG of H+ ejection decreases. The coupled reaction of electron transfer with H+ ejection becomes spontaneous. Respiration resumes or is stimulated.
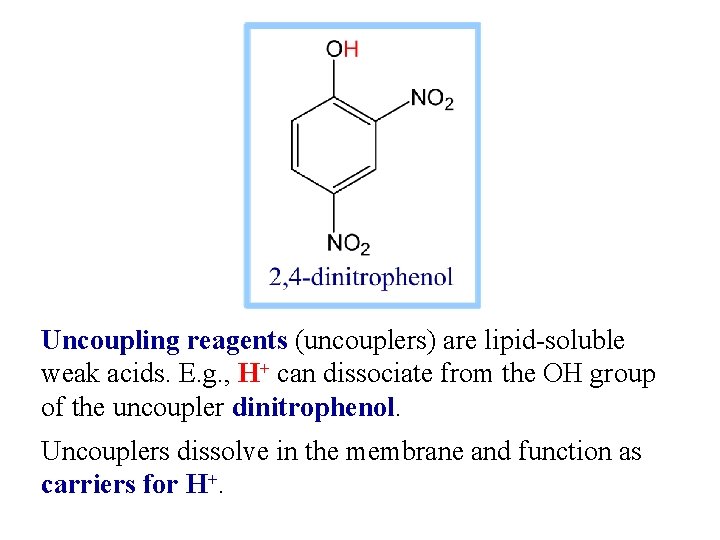
Uncoupling reagents (uncouplers) are lipid-soluble weak acids. E. g. , H+ can dissociate from the OH group of the uncoupler dinitrophenol. Uncouplers dissolve in the membrane and function as carriers for H+.
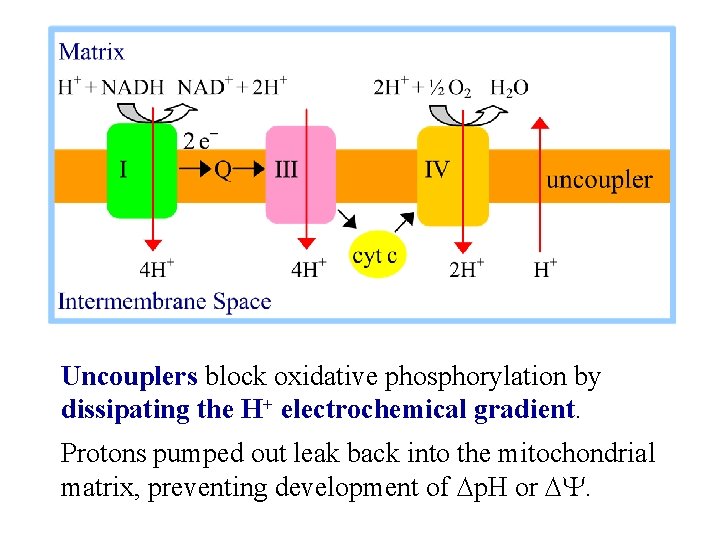
Uncouplers block oxidative phosphorylation by dissipating the H+ electrochemical gradient. Protons pumped out leak back into the mitochondrial matrix, preventing development of Dp. H or DY.
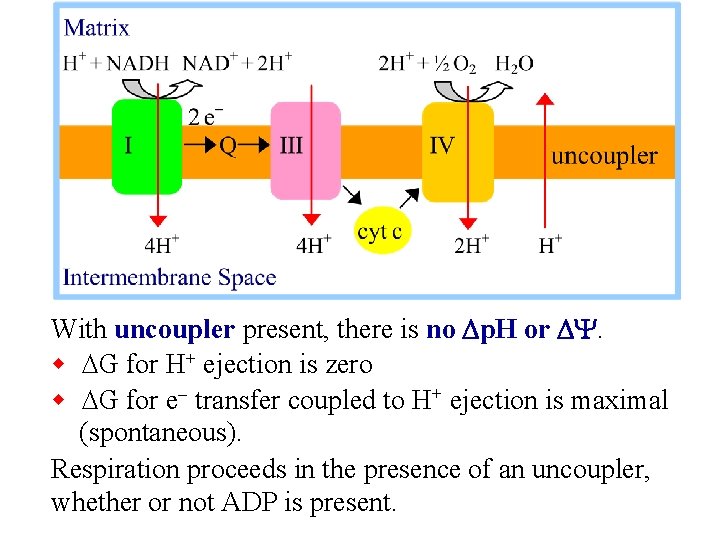
With uncoupler present, there is no Dp. H or DY. w DG for H+ ejection is zero w DG for e- transfer coupled to H+ ejection is maximal (spontaneous). Respiration proceeds in the presence of an uncoupler, whether or not ADP is present.
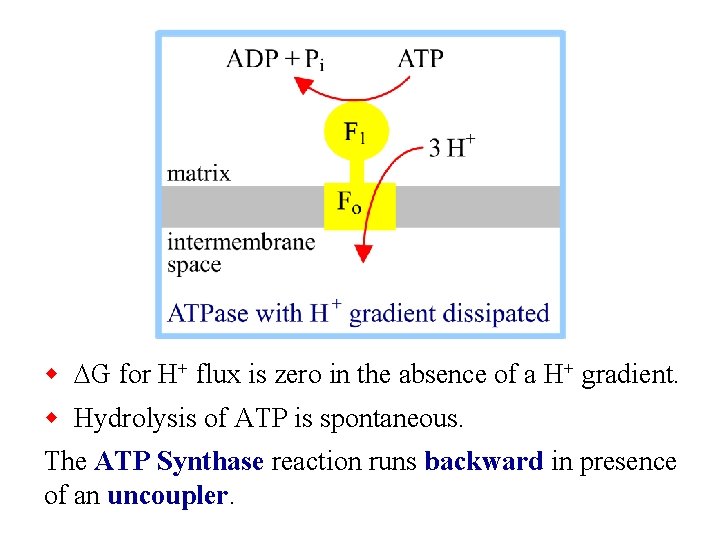
w DG for H+ flux is zero in the absence of a H+ gradient. w Hydrolysis of ATP is spontaneous. The ATP Synthase reaction runs backward in presence of an uncoupler.
Uncoupling Protein An uncoupling protein (thermogenin) is produced in brown adipose tissue of newborn mammals and hibernating mammals. This protein of the inner mitochondrial membrane functions as a H+carrier. The uncoupling protein blocks development of a H+ electrochemical gradient, thereby stimulating respiration. DG of respiration is dissipated as heat. This "non-shivering thermogenesis" is costly in terms of respiratory energy unavailable for ATP synthesis, but provides valuable warming of the organism.
- Slides: 47