Mole Lesson 2 Comparing Masses of different Substances

Mole~ Lesson 2 Comparing Masses of different Substances ITS Chemistry

SI Units of measurement Quantity Name Symbol Length Metre M Mass Kilogram Kg Time Second s Temperature Kelvin K Electric current Ampere A Luminous intensity Candela cd Amount of substance mol ITS Chemistry

The Mole ~ Measure of amount of substance ITS Chemistry
The mole • One mole of any substance always contains the same number of particles. • One mole of a substance always contains 6 x 1023 particles (atoms or molecules) • The mass of one mole of a substance is called the Relative Atomic Mass (RAM). for example: 1 mole of carbon = 12 grams ITS Chemistry

The mole This number, 6 x 1023, is called Avogadro’s constant ITS Chemistry
The mole • The number of atoms in 1 gram of hydrogen is called a mole. • 1 gram of hydrogen contains approx. 6 X 1023 atoms. • 600, 000, 000, 000 atoms – rather a lot! ITS Chemistry
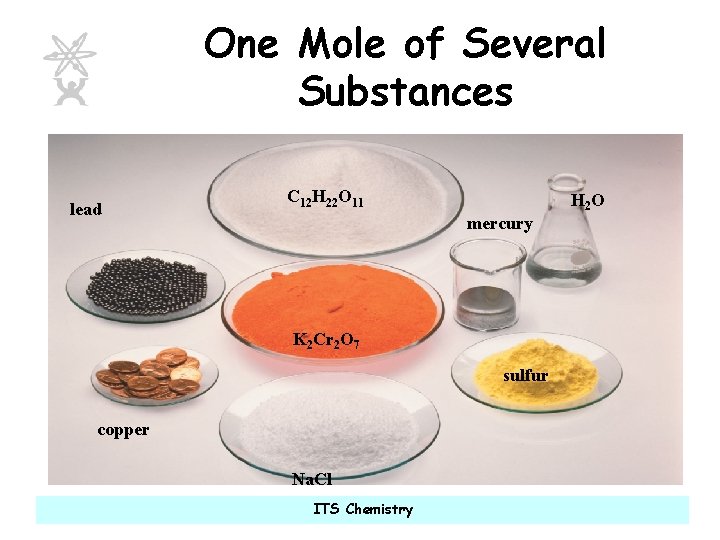
One Mole of Several Substances lead C 12 H 22 O 11 H 2 O mercury K 2 Cr 2 O 7 sulfur copper Na. Cl ITS Chemistry

What have each of these substances got in common? All of these piles contain 6 X 1023 particles ITS Chemistry

Why use moles? ? ? • It’s like cashing in 1 p coins at the bank. • If you take a thousand 1 p coins to your bank, does the cashier count out each coin? • The coins are weighed out on scales. The scales ‘know’ the mass of one hundred 1 p coins, and tell the cashier how many pounds (£s) are on the scales. ITS Chemistry

Practice Try these yourself! How many moles are in: 1. 2. 3. 4. 5. 2 g of hydrogen 36 g of carbon 160 g of oxygen 1. 4 g of nitrogen 0. 19 g of fluorine (R. A. M. s H = 1, C = 12, O = 16, N = 14, F = 19) ITS Chemistry

Answers 1. 2 g of hydrogen ~ 2 moles 2. 36 g of carbon ~ 3 moles 3. 160 g of oxygen ~ 10 moles 4. 1. 4 g of nitrogen ~ 0. 1 moles 5. 0. 19 g of fluorine ~ 0. 01 moles ITS Chemistry
- Slides: 11