Mole Empirical Formula Stoichiometry The Mole Determining the


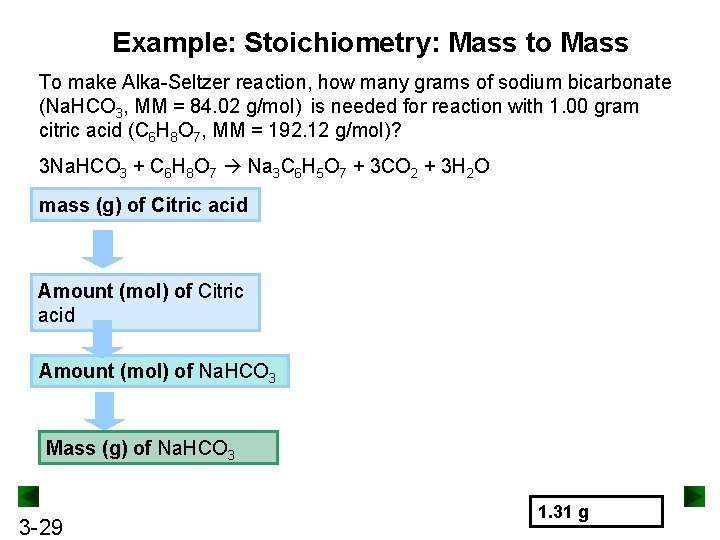





- Slides: 49

Mole, Empirical Formula, Stoichiometry The Mole Determining the Formula of an Unknown Compound Writing and Balancing Chemical Equations Calculating Quantities of Reactant and Product Solution stoichiometry 3 -1

The Mole The mole (mol) is the amount of a substance that contains the same number of entities as there atoms in exactly 12 g of carbon-12. The term “entities” refers to atoms, ions, molecules, formula units, or electrons – in fact, any type of particle. One mole (1 mol) contains 6. 022 x 1023 entities (to four significant figures). This number (N) is called Avogadro’s number. 3 -2

Molar Mass Definition: The mass per mole of its entities (atoms, molecules, or formula units). For monatomic elements, the molar mass is the same as the atomic mass in grams per mole. The atomic mass is simply read from the Periodic Table. The molar mass of Ne = 20. 18 g/mol. Practice: (NH 4)2 SO 4 132. 15 g/mol Mg. SO 4 · 7 H 2 O as Hydrate 246. 47 g/mol 3 -3

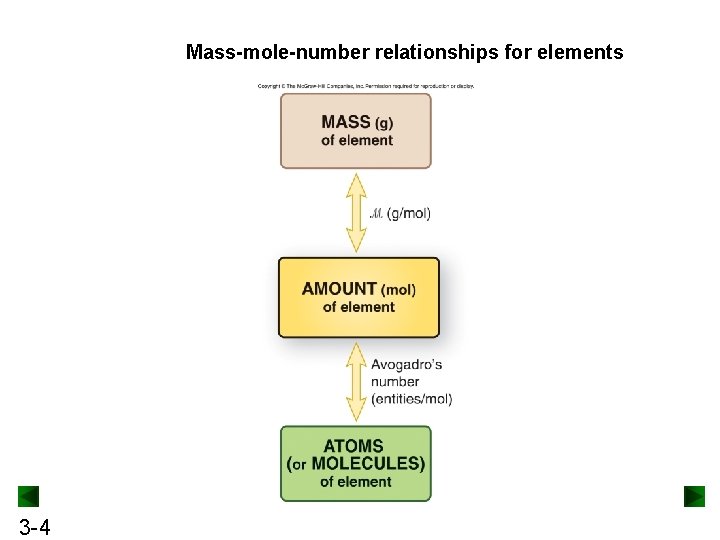
Mass-mole-number relationships for elements 3 -4

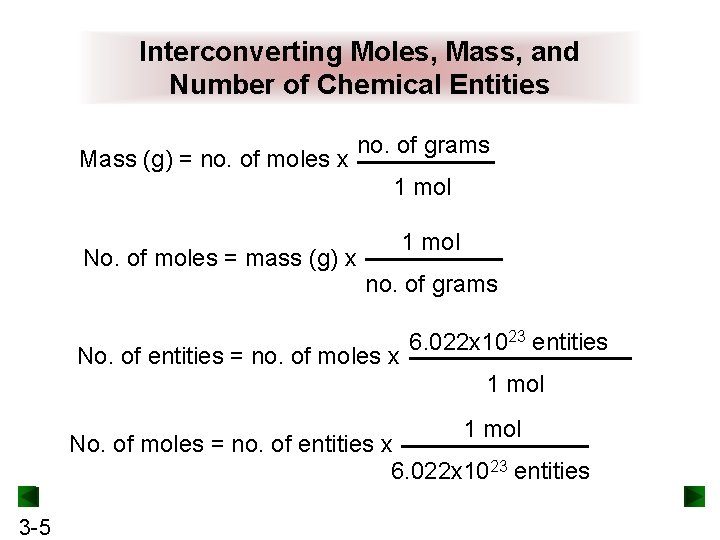
Interconverting Moles, Mass, and Number of Chemical Entities Mass (g) = no. of moles x no. of grams 1 mol No. of moles = mass (g) x 1 mol no. of grams No. of entities = no. of moles x 6. 022 x 1023 entities 1 mol No. of moles = no. of entities x 6. 022 x 1023 entities 3 -5

Practice: Converting among Mass, Mole, and #Atoms/molecules of an Element A. How many water molecules in 1. 00 m. L water? Density of water = ____ B. How many grams do 7. 5 billion carbon dioxide molecules weigh? A. 1. 58 x 1023 atoms; B. 5. 48 x 10 -13 g 3 -6

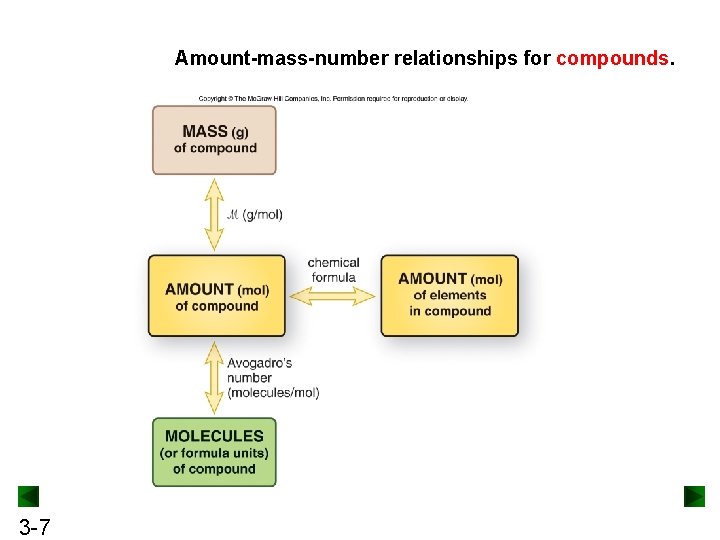
Amount-mass-number relationships for compounds. 3 -7

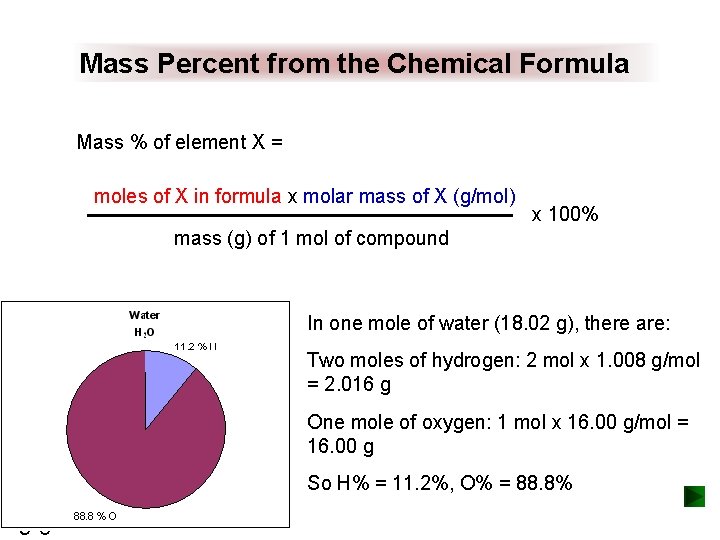
Mass Percent from the Chemical Formula Mass % of element X = moles of X in formula x molar mass of X (g/mol) x 100% mass (g) of 1 mol of compound In one mole of water (18. 02 g), there are: Two moles of hydrogen: 2 mol x 1. 008 g/mol = 2. 016 g One mole of oxygen: 1 mol x 16. 00 g/mol = 16. 00 g So H% = 11. 2%, O% = 88. 8% 3 -8

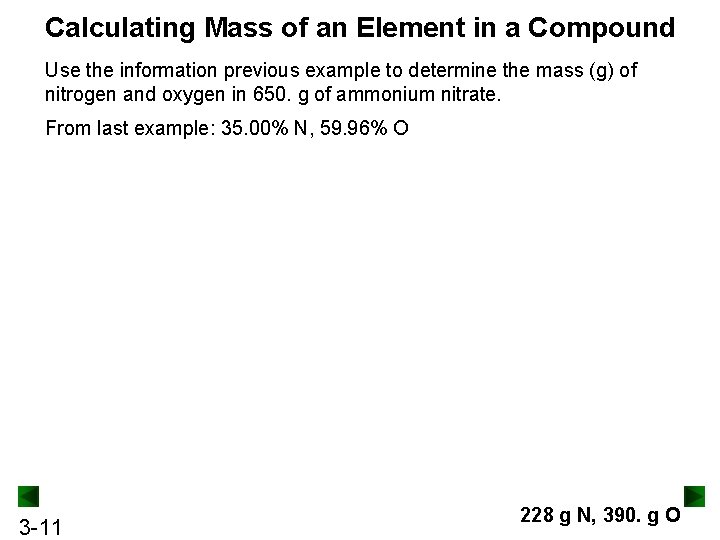
Practice: Calculating the Mass Percent of Each Element in a Compound Farmers base the effectiveness of fertilizers on their nitrogen content. Ammonium nitrate is a common fertilizer. What is the mass percent of nitrogen and oxygen in ammonium nitrate? 3 -9 35. 00 mass % N 59. 96 mass % O

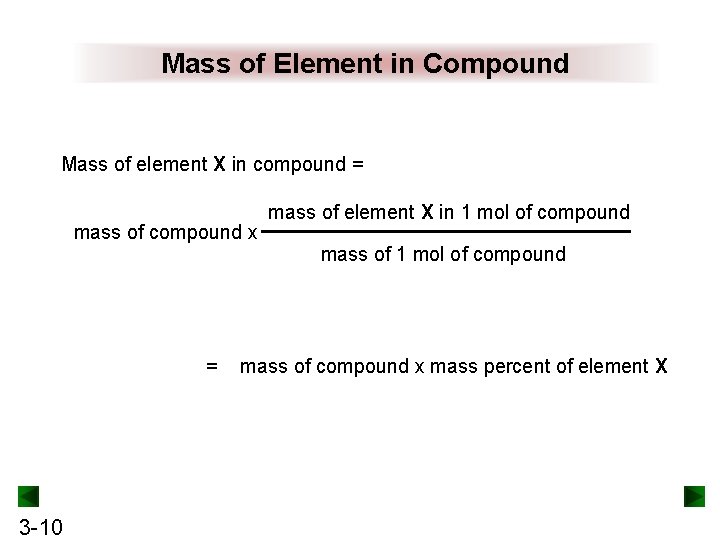
Mass of Element in Compound Mass of element X in compound = mass of compound x = 3 -10 mass of element X in 1 mol of compound mass of compound x mass percent of element X

Calculating Mass of an Element in a Compound Use the information previous example to determine the mass (g) of nitrogen and oxygen in 650. g of ammonium nitrate. From last example: 35. 00% N, 59. 96% O 3 -11 228 g N, 390. g O

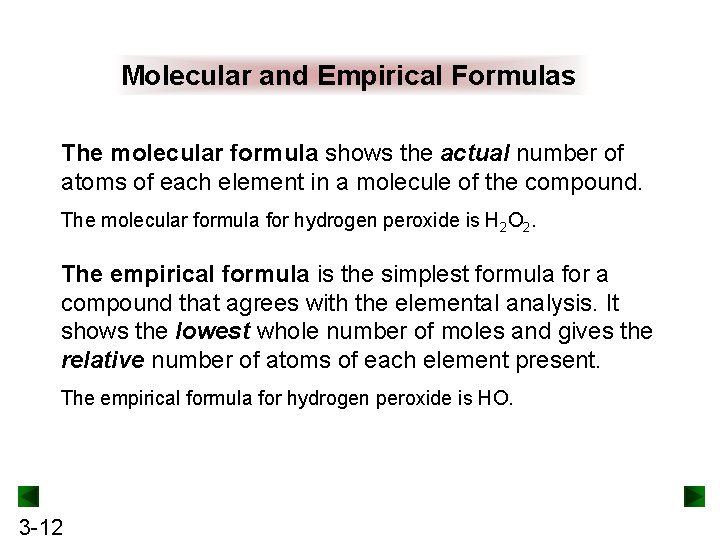
Molecular and Empirical Formulas The molecular formula shows the actual number of atoms of each element in a molecule of the compound. The molecular formula for hydrogen peroxide is H 2 O 2. The empirical formula is the simplest formula for a compound that agrees with the elemental analysis. It shows the lowest whole number of moles and gives the relative number of atoms of each element present. The empirical formula for hydrogen peroxide is HO. 3 -12

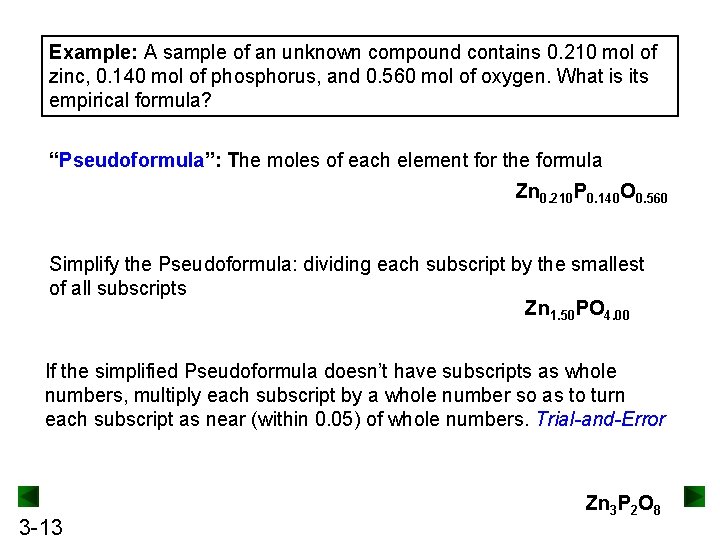
Example: A sample of an unknown compound contains 0. 210 mol of zinc, 0. 140 mol of phosphorus, and 0. 560 mol of oxygen. What is its empirical formula? “Pseudoformula”: The moles of each element for the formula Zn 0. 210 P 0. 140 O 0. 560 Simplify the Pseudoformula: dividing each subscript by the smallest of all subscripts Zn 1. 50 PO 4. 00 If the simplified Pseudoformula doesn’t have subscripts as whole numbers, multiply each subscript by a whole number so as to turn each subscript as near (within 0. 05) of whole numbers. Trial-and-Error 3 -13 Zn 3 P 2 O 8

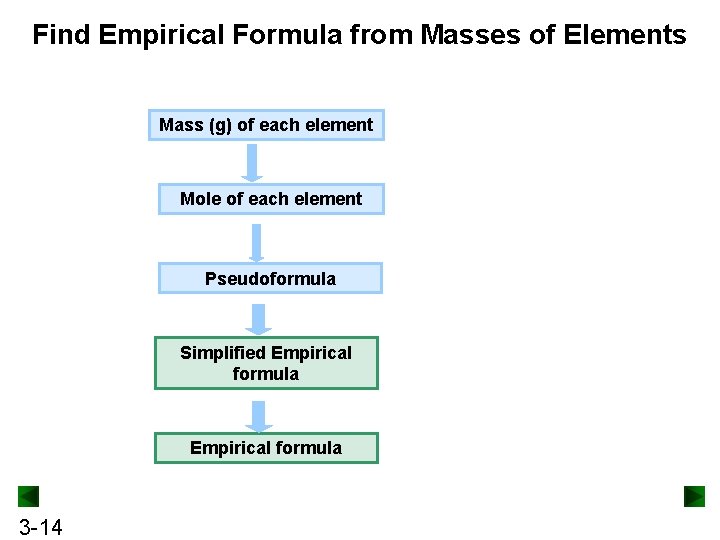
Find Empirical Formula from Masses of Elements Mass (g) of each element Mole of each element Pseudoformula Simplified Empirical formula 3 -14

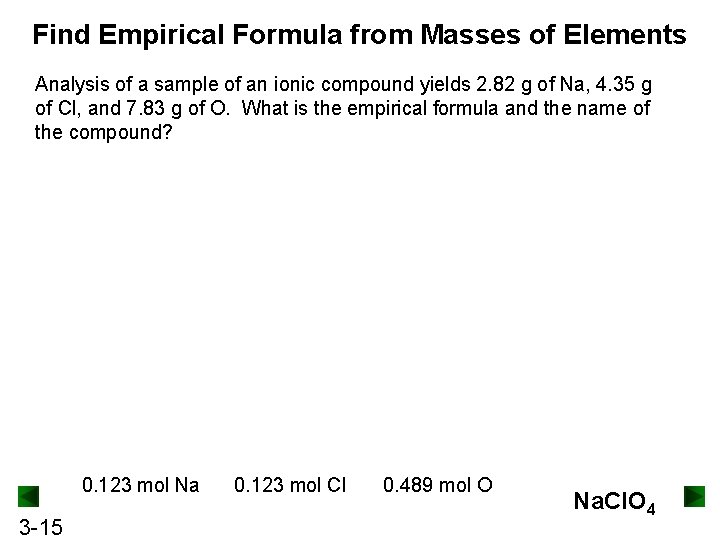
Find Empirical Formula from Masses of Elements Analysis of a sample of an ionic compound yields 2. 82 g of Na, 4. 35 g of Cl, and 7. 83 g of O. What is the empirical formula and the name of the compound? 0. 123 mol Na 3 -15 0. 123 mol Cl 0. 489 mol O Na. Cl. O 4

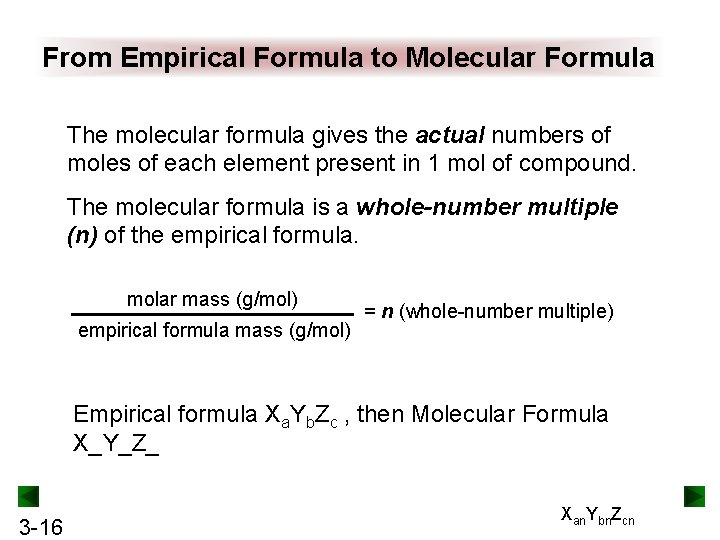
From Empirical Formula to Molecular Formula The molecular formula gives the actual numbers of moles of each element present in 1 mol of compound. The molecular formula is a whole-number multiple (n) of the empirical formula. molar mass (g/mol) empirical formula mass (g/mol) = n (whole-number multiple) Empirical formula Xa. Yb. Zc , then Molecular Formula X_Y_Z_ 3 -16 Xan. Ybn. Zcn

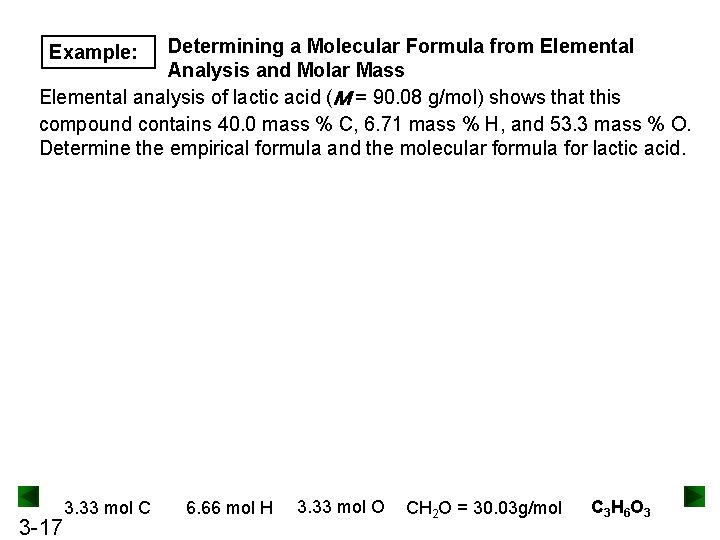
Determining a Molecular Formula from Elemental Analysis and Molar Mass Elemental analysis of lactic acid (M = 90. 08 g/mol) shows that this compound contains 40. 0 mass % C, 6. 71 mass % H, and 53. 3 mass % O. Determine the empirical formula and the molecular formula for lactic acid. Example: 3 -17 3. 33 mol C 6. 66 mol H 3. 33 mol O CH 2 O = 30. 03 g/mol C 3 H 6 O 3

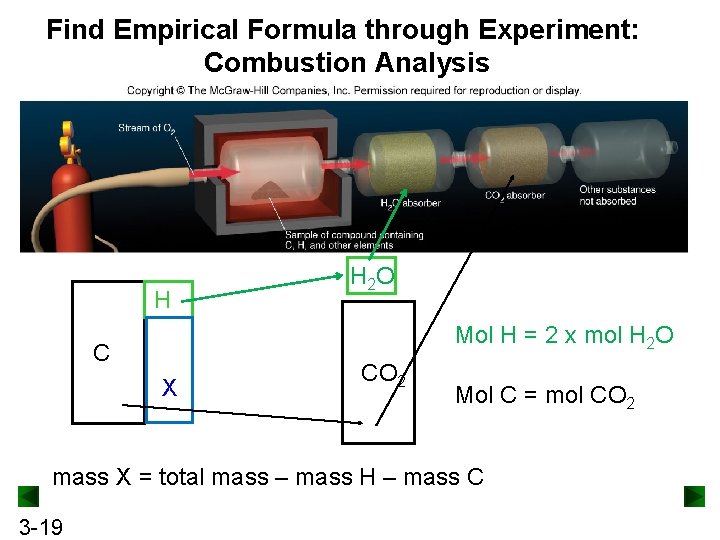
Combustion Analysis: Find Empirical Formula Combustion analysis used in chemistry to determine the elemental composition (empirical formula) of a pure organic compound (containing C, H, N, O, S, etc. ) by combusting the sample to quantitatively analyze the resulting combustion products (water, carbon dioxide, etc. ). Assuming ALL C converted to CO 2 and ALL H converted to H 2 O, the number of moles of each combustion product can be determined The mole of each element can be determined eventually, leading to the empirical formula 3 -18

Find Empirical Formula through Experiment: Combustion Analysis H H 2 O Mol H = 2 x mol H 2 O C X CO 2 Mol C = mol CO 2 mass X = total mass – mass H – mass C 3 -19

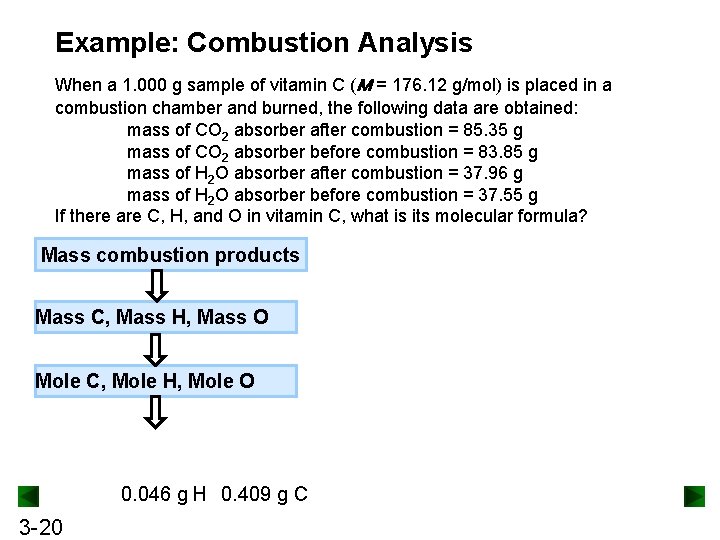
Example: Combustion Analysis When a 1. 000 g sample of vitamin C (M = 176. 12 g/mol) is placed in a combustion chamber and burned, the following data are obtained: mass of CO 2 absorber after combustion = 85. 35 g mass of CO 2 absorber before combustion = 83. 85 g mass of H 2 O absorber after combustion = 37. 96 g mass of H 2 O absorber before combustion = 37. 55 g If there are C, H, and O in vitamin C, what is its molecular formula? Mass combustion products Mass C, Mass H, Mass O Mole C, Mole H, Mole O 0. 046 g H 0. 409 g C 3 -20

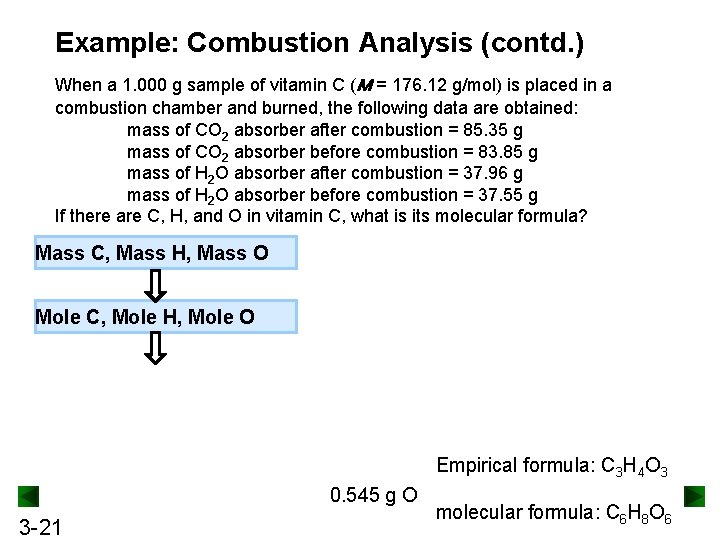
Example: Combustion Analysis (contd. ) When a 1. 000 g sample of vitamin C (M = 176. 12 g/mol) is placed in a combustion chamber and burned, the following data are obtained: mass of CO 2 absorber after combustion = 85. 35 g mass of CO 2 absorber before combustion = 83. 85 g mass of H 2 O absorber after combustion = 37. 96 g mass of H 2 O absorber before combustion = 37. 55 g If there are C, H, and O in vitamin C, what is its molecular formula? Mass C, Mass H, Mass O Mole C, Mole H, Mole O Empirical formula: C 3 H 4 O 3 0. 545 g O 3 -21 molecular formula: C 6 H 8 O 6

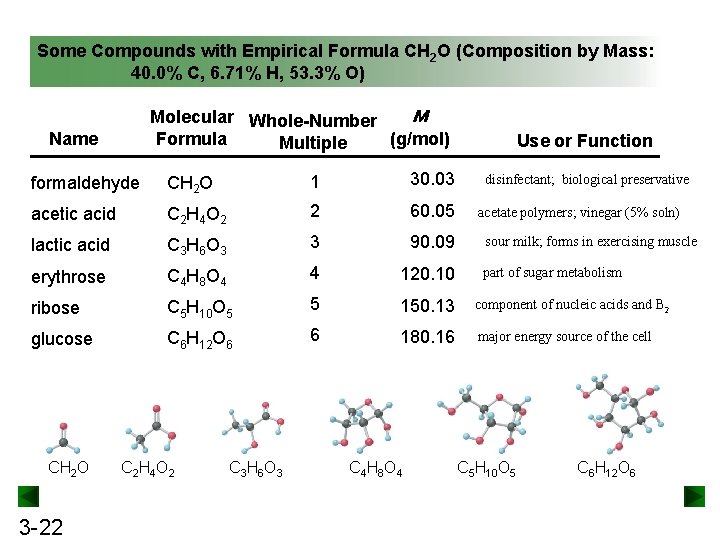
Some Compounds with Empirical Formula CH 2 O (Composition by Mass: 40. 0% C, 6. 71% H, 53. 3% O) M Molecular Whole-Number Formula (g/mol) Multiple Name Use or Function formaldehyde CH 2 O 1 30. 03 acetic acid C 2 H 4 O 2 2 60. 05 lactic acid C 3 H 6 O 3 3 90. 09 erythrose C 4 H 8 O 4 4 120. 10 ribose C 5 H 10 O 5 5 150. 13 component of nucleic acids and B 2 glucose C 6 H 12 O 6 6 180. 16 major energy source of the cell CH 2 O 3 -22 C 2 H 4 O 2 C 3 H 6 O 3 C 4 H 8 O 4 disinfectant; biological preservative acetate polymers; vinegar (5% soln) sour milk; forms in exercising muscle part of sugar metabolism C 5 H 10 O 5 C 6 H 12 O 6

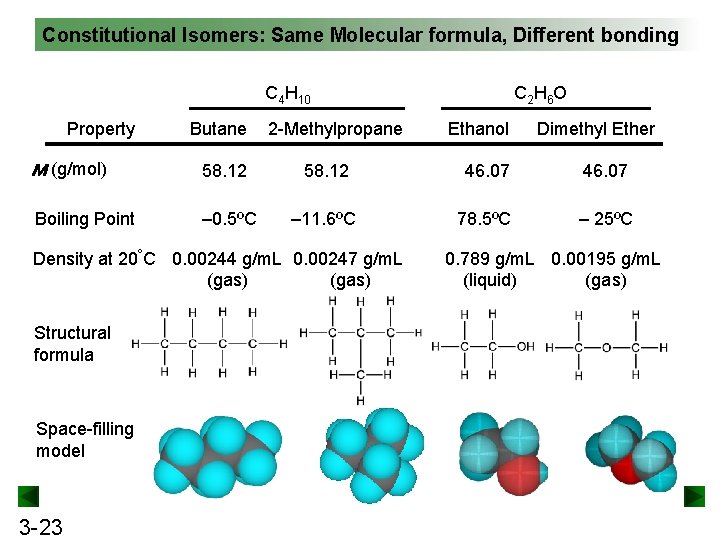
Constitutional Isomers: Same Molecular formula, Different bonding C 4 H 10 Property Butane 2 -Methylpropane C 2 H 6 O Ethanol Dimethyl Ether M (g/mol) 58. 12 46. 07 Boiling Point – 0. 5ºC – 11. 6ºC 78. 5ºC – 25ºC Density at 20°C 0. 00244 g/m. L 0. 00247 g/m. L (gas) Structural formula Space-filling model 3 -23 0. 789 g/m. L 0. 00195 g/m. L (liquid) (gas)

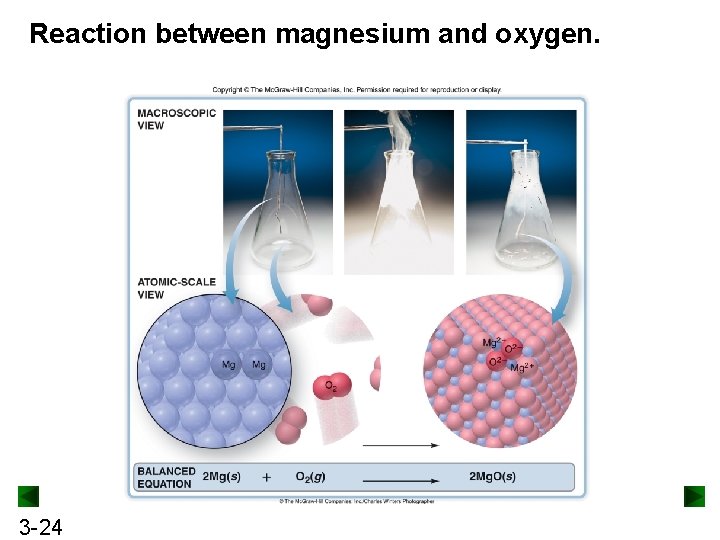
Reaction between magnesium and oxygen. 3 -24

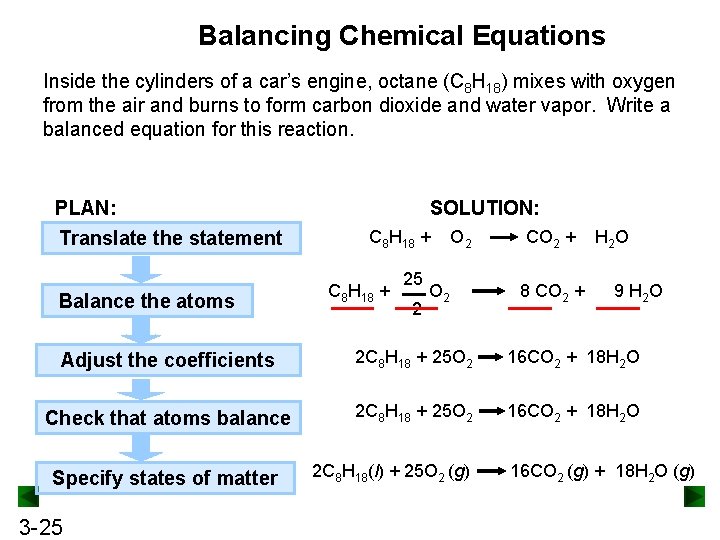
Balancing Chemical Equations Inside the cylinders of a car’s engine, octane (C 8 H 18) mixes with oxygen from the air and burns to form carbon dioxide and water vapor. Write a balanced equation for this reaction. PLAN: Translate the statement Balance the atoms SOLUTION: C 8 H 18 + 25 2 O 2 CO 2 + 8 CO 2 + H 2 O 9 H 2 O Adjust the coefficients 2 C 8 H 18 + 25 O 2 16 CO 2 + 18 H 2 O Check that atoms balance 2 C 8 H 18 + 25 O 2 16 CO 2 + 18 H 2 O Specify states of matter 3 -25 2 C 8 H 18(l) + 25 O 2 (g) 16 CO 2 (g) + 18 H 2 O (g)

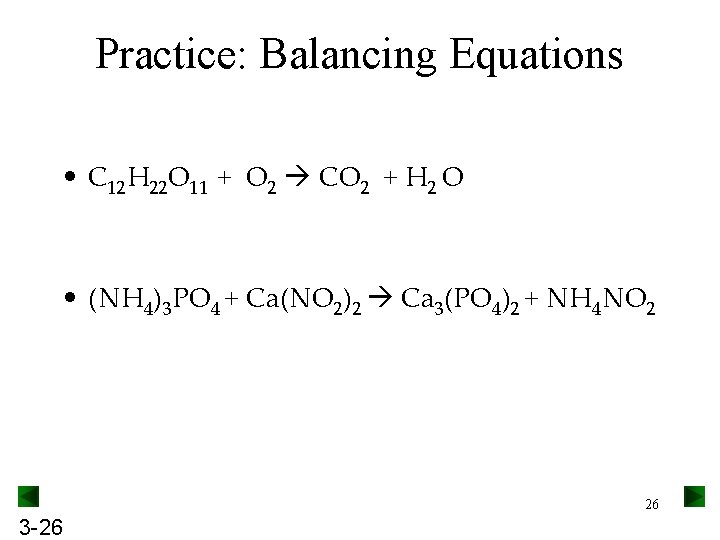
Practice: Balancing Equations • C 12 H 22 O 11 + O 2 CO 2 + H 2 O • (NH 4)3 PO 4 + Ca(NO 2)2 Ca 3(PO 4)2 + NH 4 NO 2 26 3 -26

Stoichiometric Calculations • The coefficients in a balanced chemical equation – represent the relative number of reactant and product particles – and the relative number of moles of each. • Since moles are related to mass – the equation can be used to calculate masses of reactants and/or products for a given reaction. – Example: To make homemade Alka-Seltzer, grams of citric acid and baking soda can be calculated from balanced equation • The mole ratios from the balanced equation are used as conversion factors. 3 -27

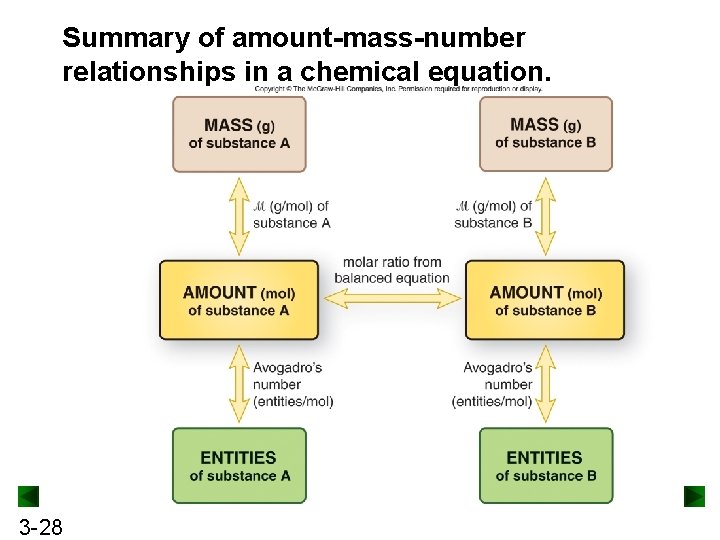
Summary of amount-mass-number relationships in a chemical equation. 3 -28
Example: Stoichiometry: Mass to Mass To make Alka-Seltzer reaction, how many grams of sodium bicarbonate (Na. HCO 3, MM = 84. 02 g/mol) is needed for reaction with 1. 00 gram citric acid (C 6 H 8 O 7, MM = 192. 12 g/mol)? 3 Na. HCO 3 + C 6 H 8 O 7 Na 3 C 6 H 5 O 7 + 3 CO 2 + 3 H 2 O mass (g) of Citric acid Amount (mol) of Na. HCO 3 Mass (g) of Na. HCO 3 3 -29 1. 31 g

Limiting Reactants (L. R. ) • So far we have assumed that reactants are present in the correct amounts to react completely. • In reality, one reactant may limit the amount of product that can form. • The limiting reactant will be completely used up in the reaction. • The reactant that is not limiting is in excess – some of this reactant will be left over. • To identify the limiting reagent, calculate the amount of one product from each of the reactant: the reactant giving the least amount of product is the L. R. 3 -30

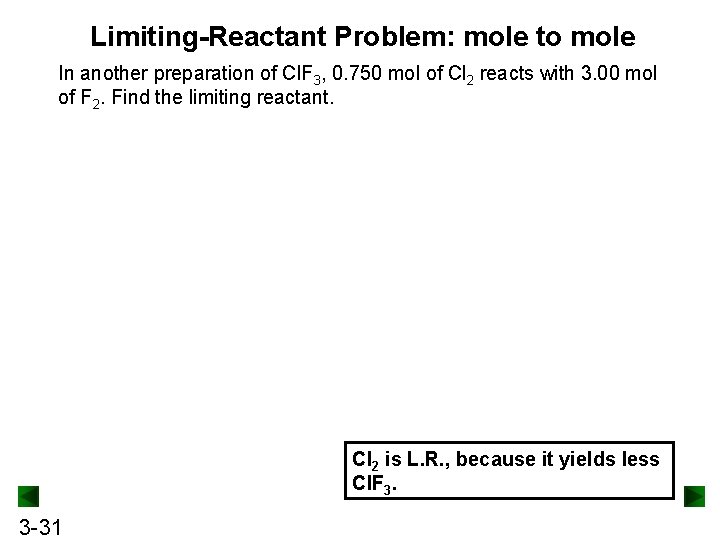
Limiting-Reactant Problem: mole to mole In another preparation of Cl. F 3, 0. 750 mol of Cl 2 reacts with 3. 00 mol of F 2. Find the limiting reactant. Cl 2 is L. R. , because it yields less Cl. F 3. 3 -31

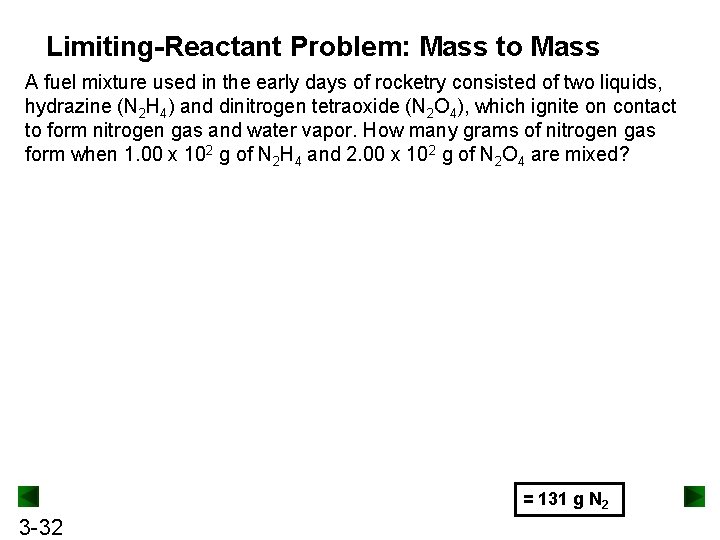
Limiting-Reactant Problem: Mass to Mass A fuel mixture used in the early days of rocketry consisted of two liquids, hydrazine (N 2 H 4) and dinitrogen tetraoxide (N 2 O 4), which ignite on contact to form nitrogen gas and water vapor. How many grams of nitrogen gas form when 1. 00 x 102 g of N 2 H 4 and 2. 00 x 102 g of N 2 O 4 are mixed? = 131 g N 2 3 -32

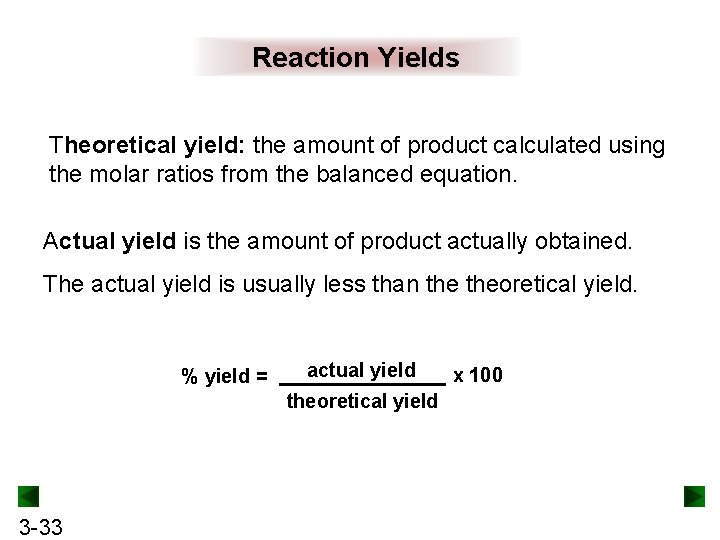
Reaction Yields Theoretical yield: the amount of product calculated using the molar ratios from the balanced equation. Actual yield is the amount of product actually obtained. The actual yield is usually less than theoretical yield. % yield = actual yield theoretical yield 3 -33 x 100

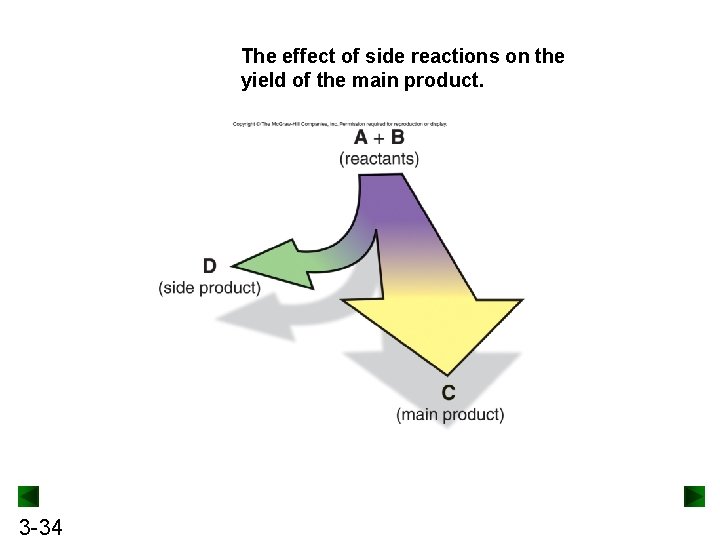
The effect of side reactions on the yield of the main product. 3 -34

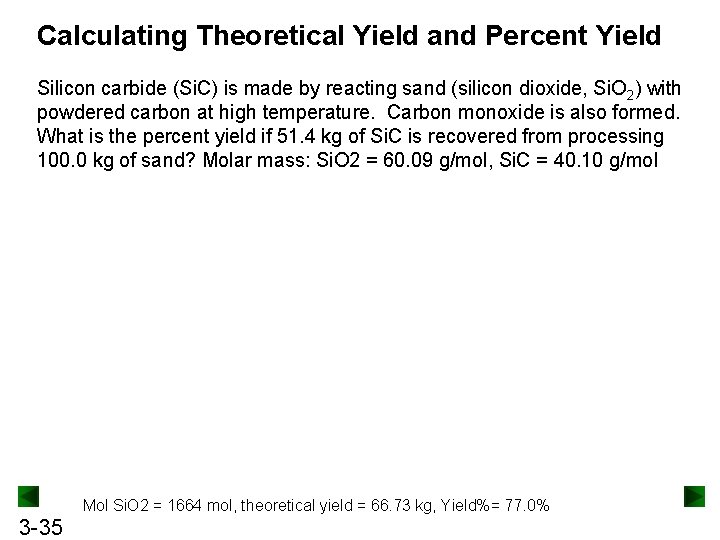
Calculating Theoretical Yield and Percent Yield Silicon carbide (Si. C) is made by reacting sand (silicon dioxide, Si. O 2) with powdered carbon at high temperature. Carbon monoxide is also formed. What is the percent yield if 51. 4 kg of Si. C is recovered from processing 100. 0 kg of sand? Molar mass: Si. O 2 = 60. 09 g/mol, Si. C = 40. 10 g/mol Mol Si. O 2 = 1664 mol, theoretical yield = 66. 73 kg, Yield%= 77. 0% 3 -35

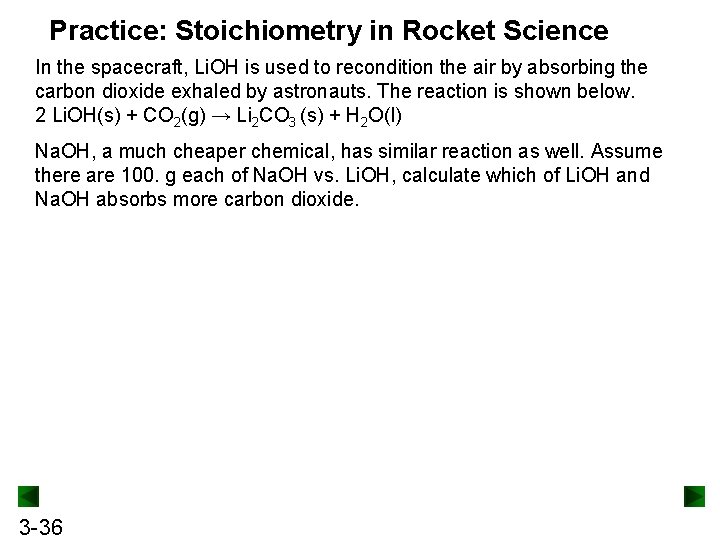
Practice: Stoichiometry in Rocket Science In the spacecraft, Li. OH is used to recondition the air by absorbing the carbon dioxide exhaled by astronauts. The reaction is shown below. 2 Li. OH(s) + CO 2(g) → Li 2 CO 3 (s) + H 2 O(l) Na. OH, a much cheaper chemical, has similar reaction as well. Assume there are 100. g each of Na. OH vs. Li. OH, calculate which of Li. OH and Na. OH absorbs more carbon dioxide. 3 -36

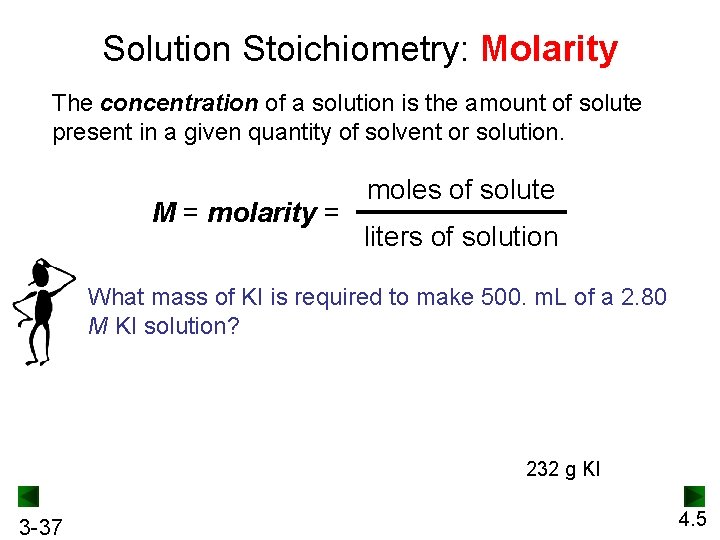
Solution Stoichiometry: Molarity The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. M = molarity = moles of solute liters of solution What mass of KI is required to make 500. m. L of a 2. 80 M KI solution? 232 g KI 3 -37 4. 5

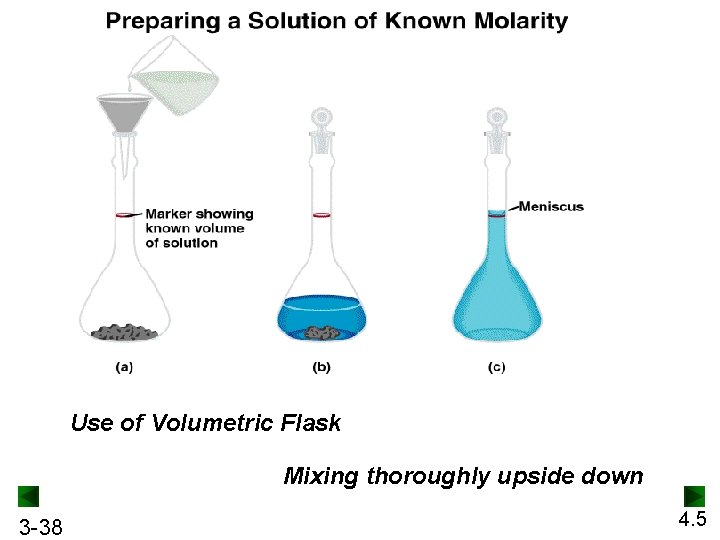
Use of Volumetric Flask Mixing thoroughly upside down 3 -38 4. 5

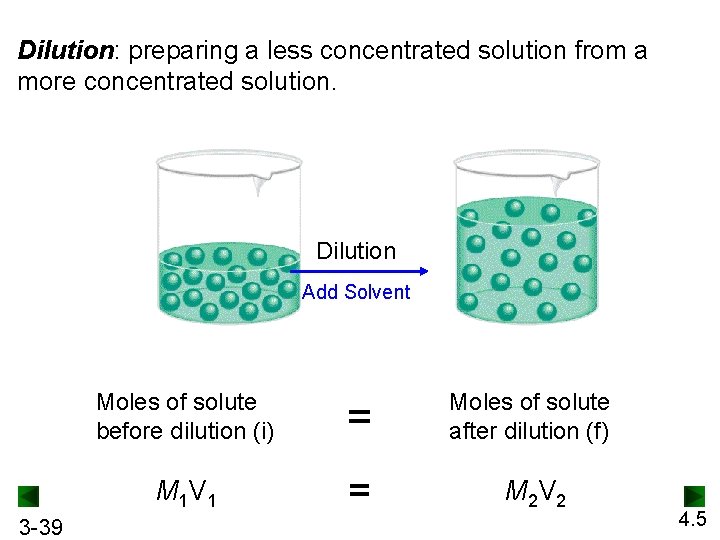
Dilution: preparing a less concentrated solution from a more concentrated solution. Dilution Add Solvent 3 -39 Moles of solute before dilution (i) = Moles of solute after dilution (f) M 1 V 1 = M 2 V 2 4. 5

Using Dilution to Prepare solutions: How would you prepare 60. 0 m. L of 0. 200 M HNO 3 from a stock solution of 4. 00 M HNO 3? 3. 00 m. L 4. 00 M HNO 3 3 -40 4. 5

Solution Stoichiometry: Gravimetric Analysis Purpose: Find the amount of solid formed in solution reactions 1. Reaction in solution creates precipitate 2. Filter and dry precipitate 3. Weigh precipitate 4. Use chemical formula and mass of precipitate to determine amount of unknown ion 3 -41 4. 6

Solution Stoichiometry: Silver nitrate reacts with sodium carbonate to form insoluble silver carbonate. Given enough sodium carbonate, what volume of 0. 100 M Silver nitrate would produce 1. 000 g silver carbonate? 18. 1 m. L 3 -42 4. 7

Solution Stoichiometry with limiting reagent: Mercury(II) ions are poisonous. To remove Hg 2+ ion from solution, soluble sulfide salt are often used to form insoluble Hg. S. If 100. m. L 1. 10 M sodium sulfide is added to 500. m. L 0. 200 M mercury(II) nitrate solution, predict theoretical yield of Hg. S in grams. 23. 3 g Hg. S 3 -43 4. 7

Solution Stoichiometry: Titration 25. 00 m. L unknown sulfuric acid solution is titrated with 0. 0987 M sodium hydroxide solution. If 23. 45 m. L of sodium hydroxide solution is used to reach endpoint, what is the molarity of sulfuric acid solution. Write balanced equation first. 3 -44 4. 7

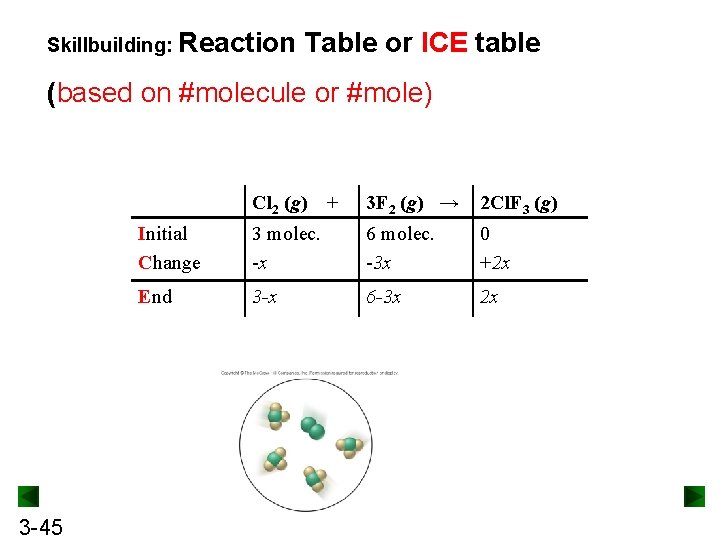
Skillbuilding: Reaction Table or ICE table (based on #molecule or #mole) 3 -45 Cl 2 (g) + 3 F 2 (g) → 2 Cl. F 3 (g) Initial Change 3 molec. -x 6 molec. -3 x 0 +2 x End 3 -x 6 -3 x 2 x

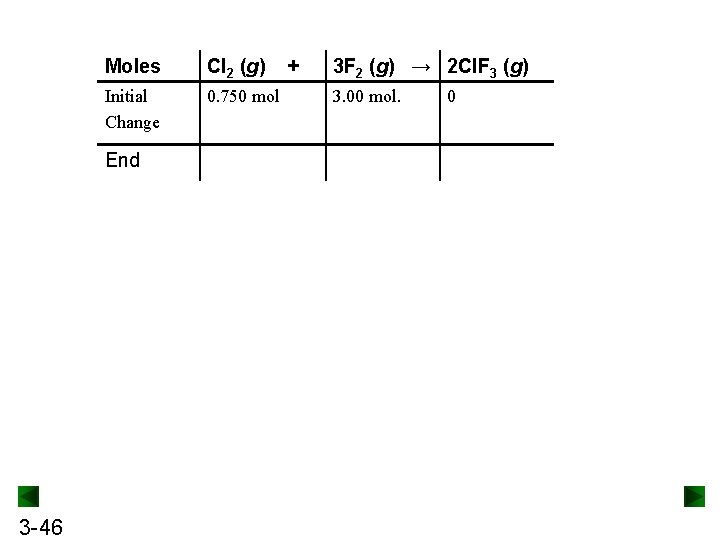
Moles Cl 2 (g) Initial Change 0. 750 mol End 3 -46 + 3 F 2 (g) → 2 Cl. F 3 (g) 3. 00 mol. 0

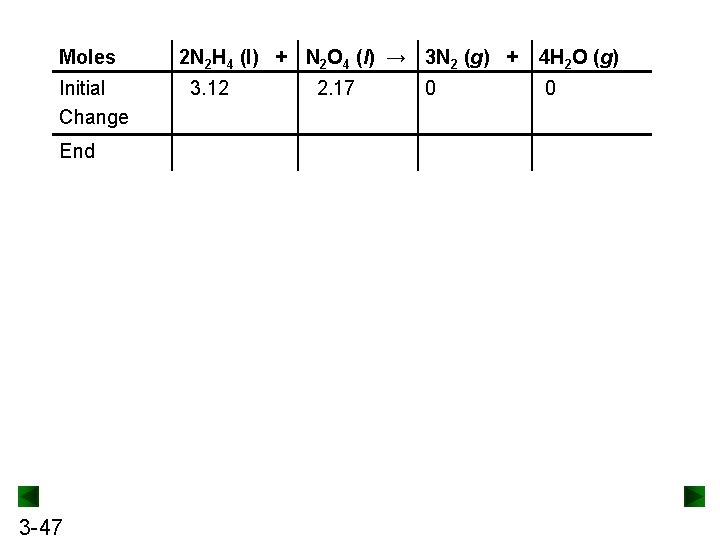
Moles Initial Change End 3 -47 2 N 2 H 4 (l) + N 2 O 4 (l) → 3 N 2 (g) + 3. 12 2. 17 0 4 H 2 O (g) 0

Example: Writing an Overall Equation for a Reaction Sequence Roasting is the first step in extracting copper from chalcocite, the ore used in the previous problem. In the next step, copper(I) oxide reacts with powdered carbon to yield copper metal and carbon monoxide gas. Write a balanced overall equation for the two-step process. 2 Cu 2 S (s) + 3 O 2 (g) + 2 C (s) → 2 SO 2 (g) + 4 Cu (s) + 2 CO (g) 3 -48

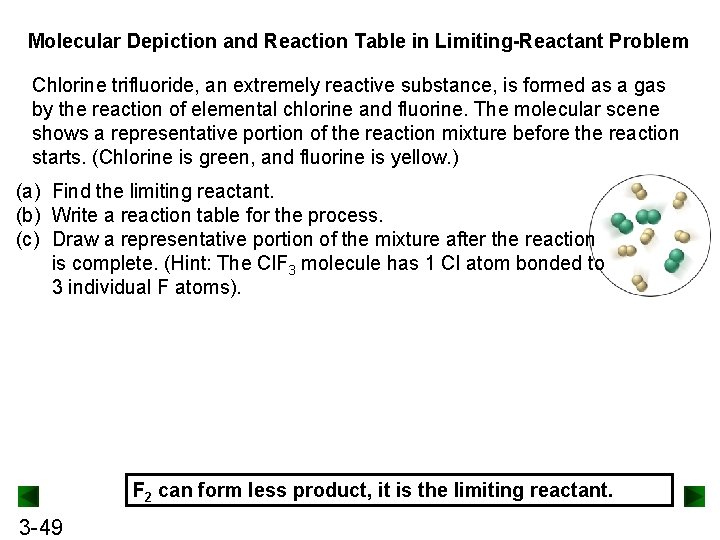
Molecular Depiction and Reaction Table in Limiting-Reactant Problem Chlorine trifluoride, an extremely reactive substance, is formed as a gas by the reaction of elemental chlorine and fluorine. The molecular scene shows a representative portion of the reaction mixture before the reaction starts. (Chlorine is green, and fluorine is yellow. ) (a) Find the limiting reactant. (b) Write a reaction table for the process. (c) Draw a representative portion of the mixture after the reaction is complete. (Hint: The Cl. F 3 molecule has 1 Cl atom bonded to 3 individual F atoms). F 2 can form less product, it is the limiting reactant. 3 -49