Molarity What is Molarity Molarity means concentration Concentration






- Slides: 13

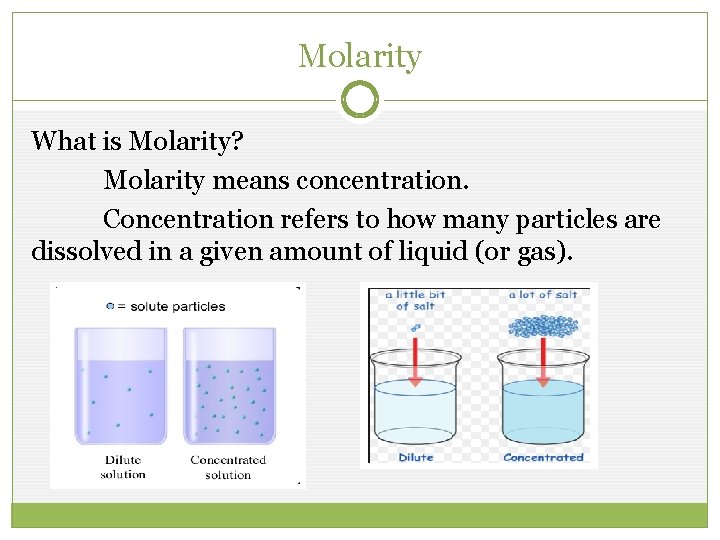
Molarity What is Molarity? Molarity means concentration. Concentration refers to how many particles are dissolved in a given amount of liquid (or gas).

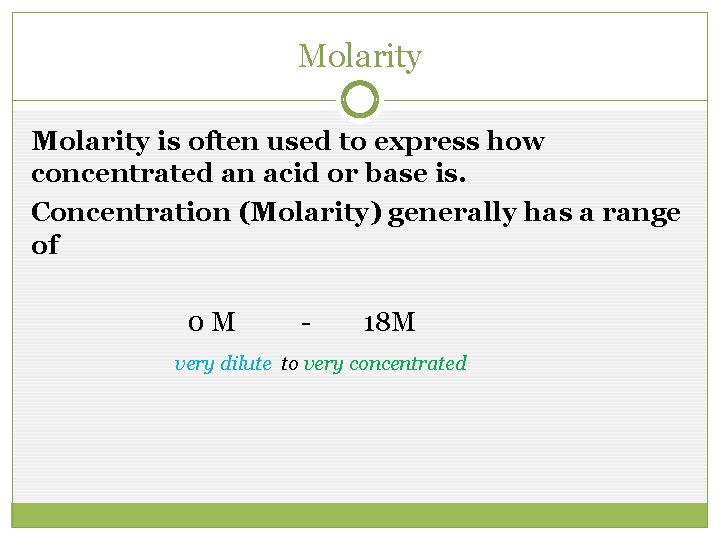
Molarity is often used to express how concentrated an acid or base is. Concentration (Molarity) generally has a range of 0 M - 18 M very dilute to very concentrated

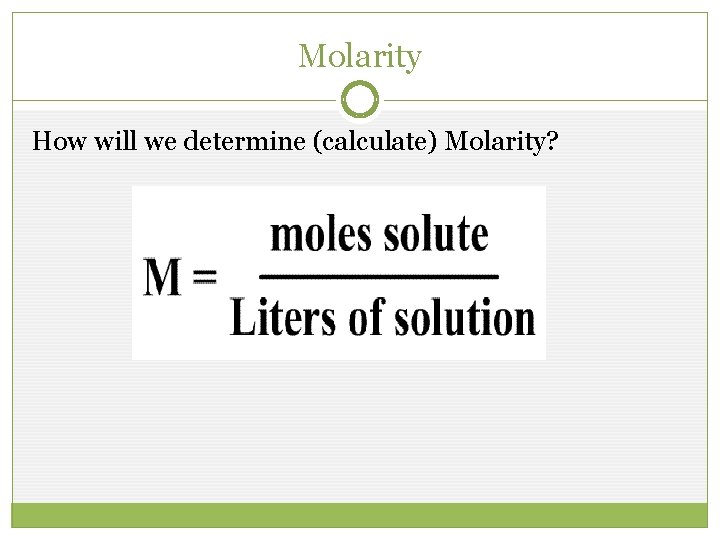
Molarity How will we determine (calculate) Molarity?

Molarity Practice: 1. If 0. 4 moles KOH is dissolved in water to make 2 L of solution, what is the molarity? Is it a concentrated/dilute and is it acid/base solution? 2. If 0. 1 mole Na. OH is dissolved in water to make 0. 05 L of solution, what is the molarity? Is it a concentrated/dilute and is it acid/base solution?

Molarity What if moles are not given? l 3. If 0. 60 grams of Na. OH is dissolved in 0. 800 L of water, what is the molarity? Calculate the p. H. 4. If 0. 20 grams of KOH is dissolved in 0. 300 L of water, what is the molarity? Calculate the p. H.

Apply what you have learned: § Match each solution with its correct description: ___1. dilute, weak acid ___2. dilute strong base ___3. concentrated, strong acid ___4. dilute strong acid ___5. concentrated, weak base A. 18 M H 2 SO 4 B. 0. 5 M Na. OH C. 15 M NH 3 D. 0. 1 M HC 2 H 3 O 2 E. 0. 1 M HCl

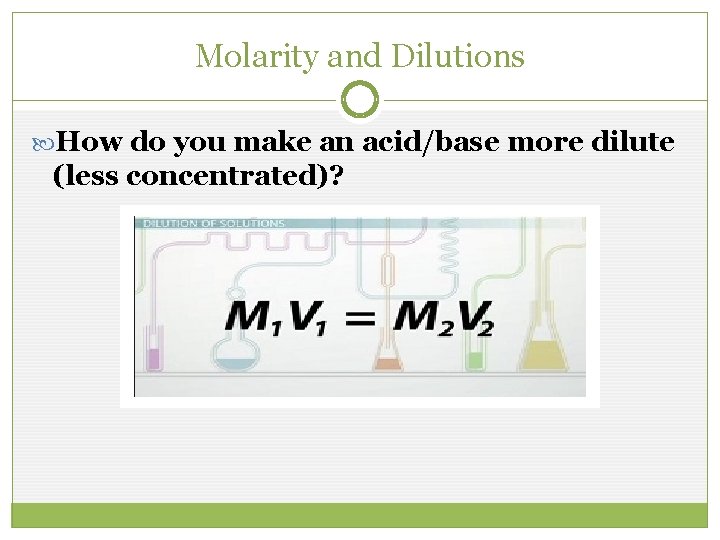
Molarity and Dilutions How do you make an acid/base more dilute (less concentrated)?

Molarity and Dilutions Ex 1: You need to prepare 300 m. L of a 0. 5 M solution of Na. OH. The only Na. OH available is 2 M. How do you make the solution?

Molarity and Dilution Lab We will use a concentrated solution of Cu. SO 4, and prepare 5 dilute solutions. What is the purpose of the 5 dilute solutions? The purpose is to accurately identify the concentrations of the 5 dilute solutions, so that we can make a calibration curve.

Molarity and Dilution What is a calibration curve? Will come from dilutions that you will do in lab A graph. Our graph will be Concentration vs Absorbance. Will come from measurement using Spectrophotometer What is the purpose of the calibration curve? To determine the concentration of an unknown quantity.

Molarity and Dilution Example: Five solutions that the concentration will be calculated and known; Solution of Unknown Concentration? ? ?

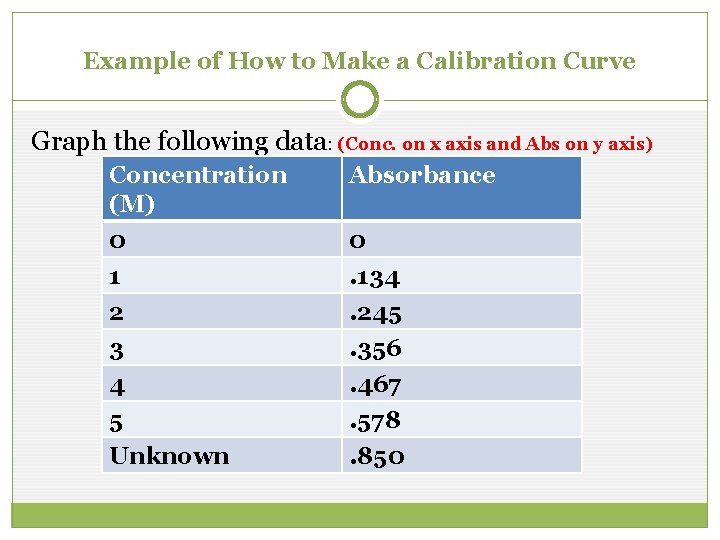
Example of How to Make a Calibration Curve Graph the following data: (Conc. on x axis and Abs on y axis) Concentration (M) 0 1 2 3 4 5 Unknown Absorbance 0. 134. 245. 356. 467. 578. 850

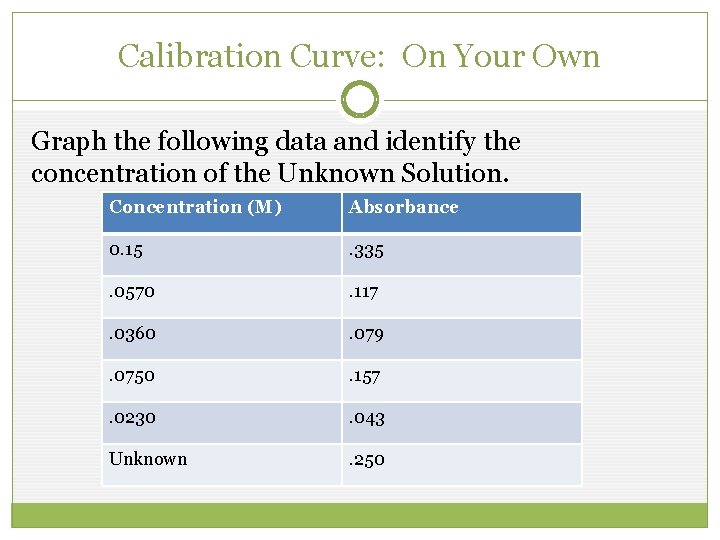
Calibration Curve: On Your Own Graph the following data and identify the concentration of the Unknown Solution. Concentration (M) Absorbance 0. 15 . 335 . 0570 . 117 . 0360 . 079 . 0750 . 157 . 0230 . 043 Unknown . 250