Molarity M State the ratio between the number
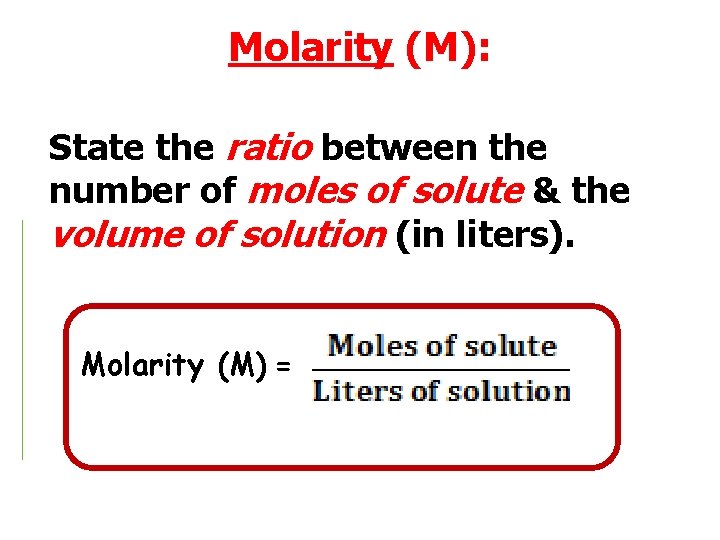
Molarity (M): State the ratio between the number of moles of solute & the volume of solution (in liters). Molarity (M) =
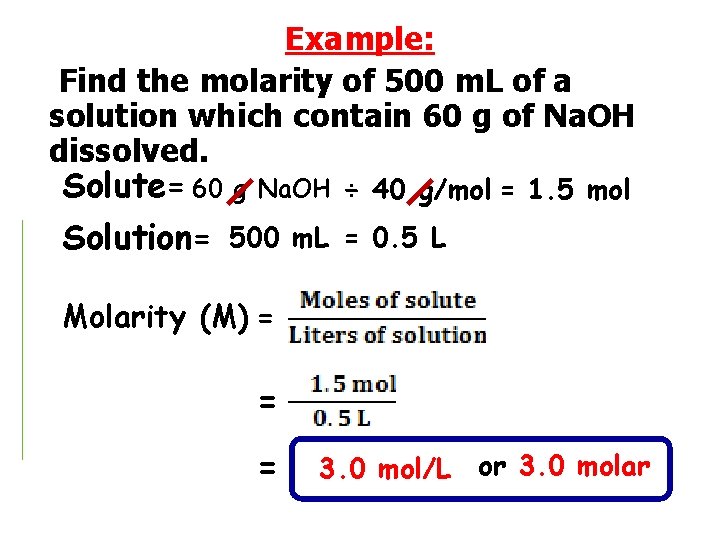
Example: Find the molarity of 500 m. L of a solution which contain 60 g of Na. OH dissolved. Solute= 60 g Na. OH ÷ 40 g/mol = 1. 5 mol Solution= 500 m. L = 0. 5 L Molarity (M) = = = 3. 0 mol/L or 3. 0 molar
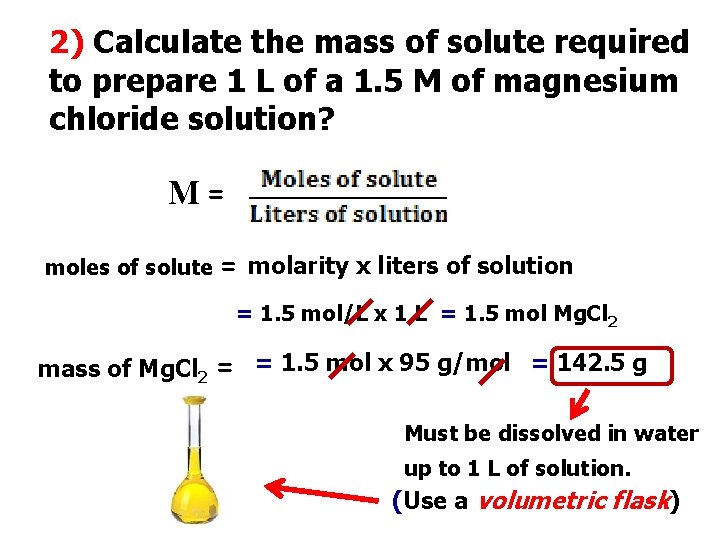
2) Calculate the mass of solute required to prepare 1 L of a 1. 5 M of magnesium chloride solution? M= moles of solute = molarity x liters of solution = 1. 5 mol/L x 1 L = 1. 5 mol Mg. Cl 2 mass of Mg. Cl 2 = = 1. 5 mol x 95 g/mol = 142. 5 g Must be dissolved in water up to 1 L of solution. (Use a volumetric flask)

Dilution: • Dilution is adding extra solvent to decrease the concentration of a solution • Dilution Formula: M 1 x V 1 = M 2 x V 2 4
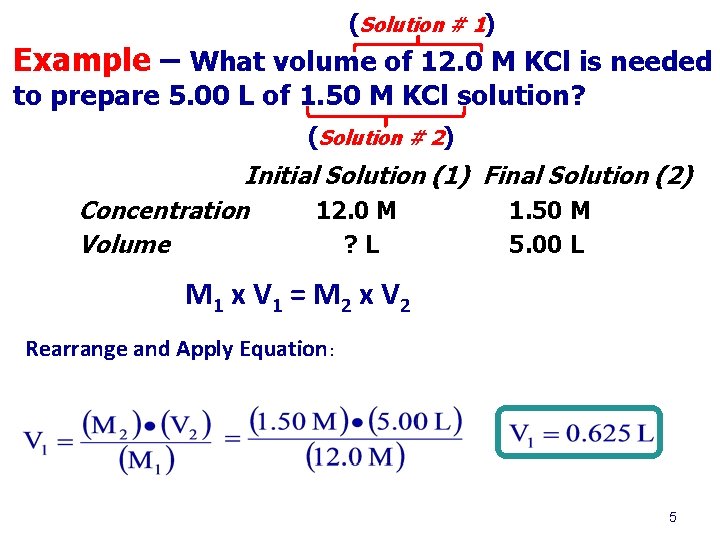
(Solution # 1) Example – What volume of 12. 0 M KCl is needed to prepare 5. 00 L of 1. 50 M KCl solution? (Solution # 2) Initial Solution (1) Final Solution (2) Concentration 12. 0 M 1. 50 M Volume ? L 5. 00 L M 1 x V 1 = M 2 x V 2 Rearrange and Apply Equation: 5
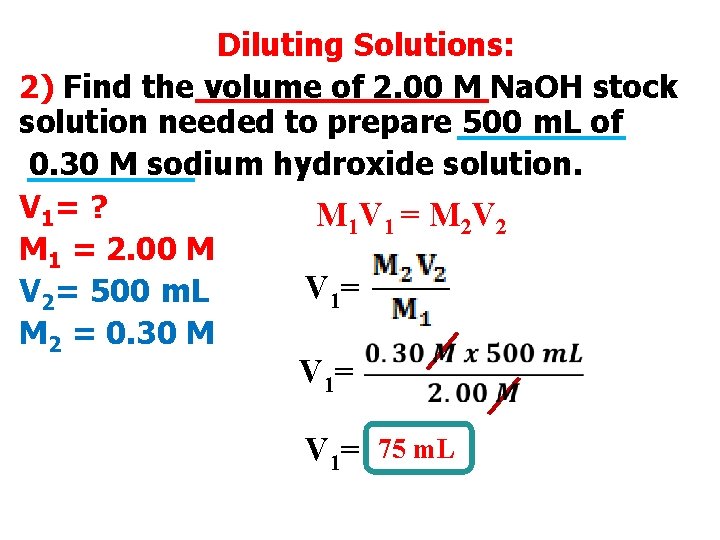
Diluting Solutions: 2) Find the volume of 2. 00 M Na. OH stock solution needed to prepare 500 m. L of 0. 30 M sodium hydroxide solution. V 1= ? M 1 V 1 = M 2 V 2 M 1 = 2. 00 M V 1= V 2= 500 m. L M 2 = 0. 30 M V 1= 75 m. L
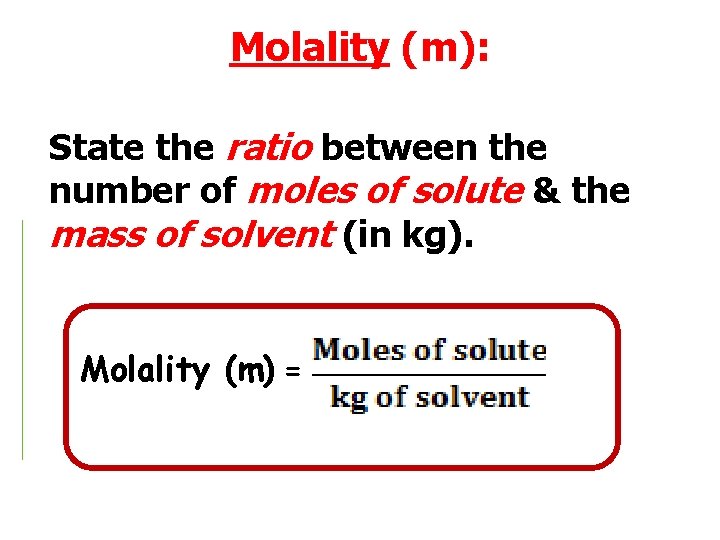
Molality (m): State the ratio between the number of moles of solute & the mass of solvent (in kg). Molality (m) =
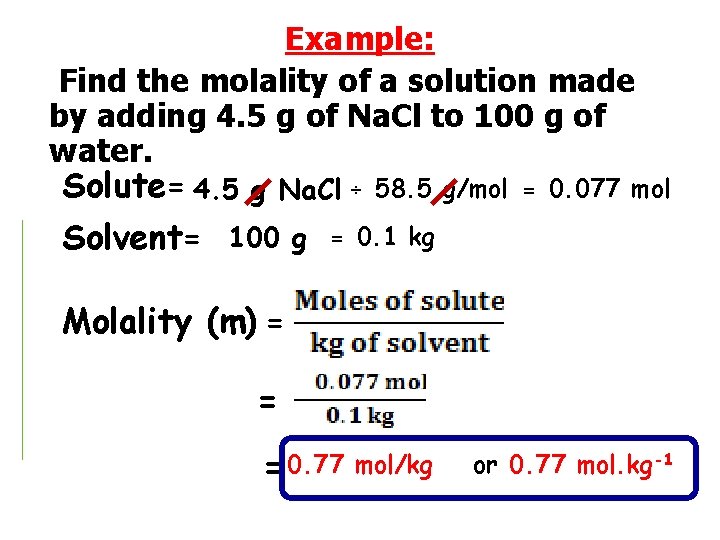
Example: Find the molality of a solution made by adding 4. 5 g of Na. Cl to 100 g of water. Solute= 4. 5 g Na. Cl ÷ 58. 5 g/mol Solvent= 100 g = 0. 1 kg = 0. 077 mol Molality (m) = = = 0. 77 mol/kg or 0. 77 mol. kg-1
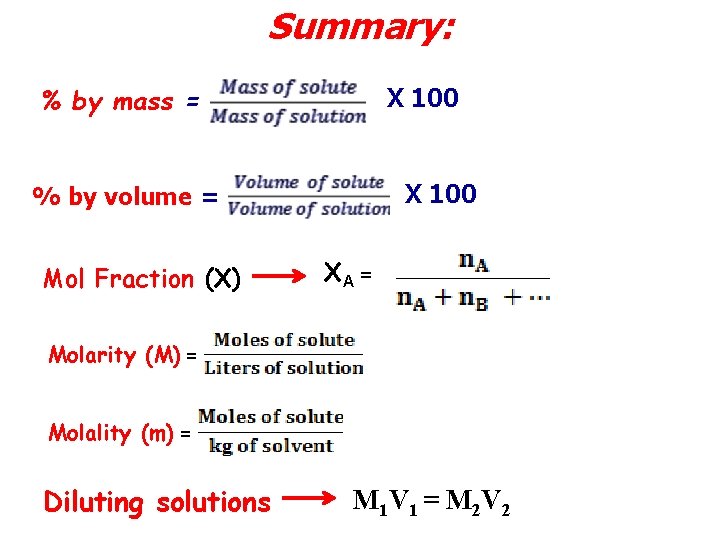
Summary: % by mass = X 100 % by volume = Mol Fraction (X) ΧA = Molarity (M) = Molality (m) = Diluting solutions M 1 V 1 = M 2 V 2
- Slides: 9