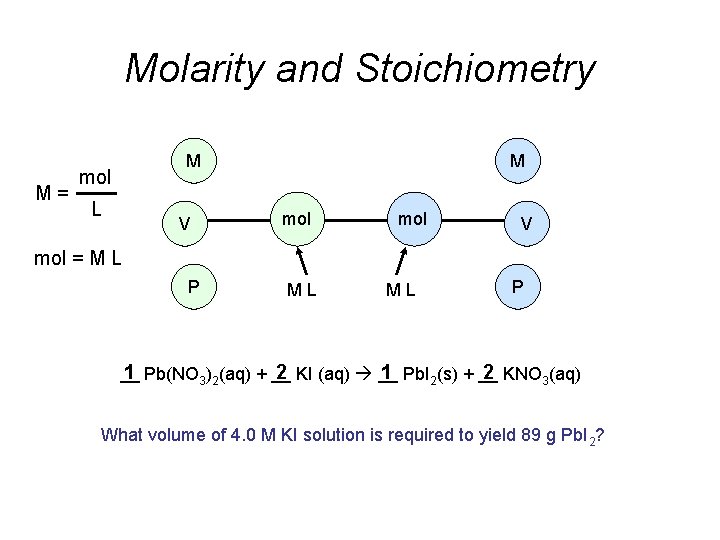
Molarity and Stoichiometry M M mol L V

- Slides: 9

Molarity and Stoichiometry M= M mol L V M mol V mol = M L P ML ML P __ 1 Pb(NO 3)2(aq) + __ 2 KI (aq) __ 1 Pb. I 2(s) + __ 2 KNO 3(aq) What volume of 4. 0 M KI solution is required to yield 89 g Pb. I 2?

Stoichiometry for Reactions in Solution Step 1) Identify the species present in the combined solution, and determine what reaction occurs. Step 2) Write the balanced net ionic equation for the reaction. Step 3) Calculate the moles of reactants. Step 4) Determine which reactant is limiting. Step 5) Calculate the moles of product or products, as required. Step 6) Convert to grams or other units, as required. Stoichiometry steps for reactions in solution

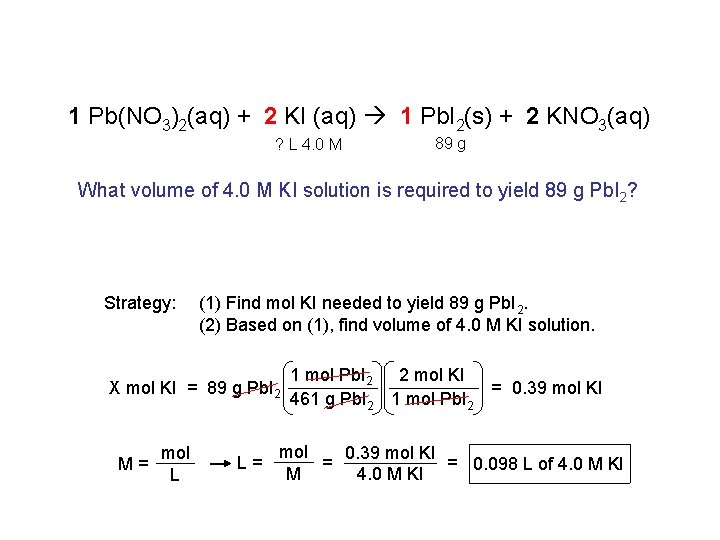
1 Pb(NO 3)2(aq) + 2 KI (aq) 1 Pb. I 2(s) + 2 KNO 3(aq) ? L 4. 0 M 89 g What volume of 4. 0 M KI solution is required to yield 89 g Pb. I 2? Strategy: (1) Find mol KI needed to yield 89 g Pb. I 2. (2) Based on (1), find volume of 4. 0 M KI solution. X mol KI = 89 g Pb. I 2 M= mol L L= 1 mol Pb. I 2 2 mol KI 461 g Pb. I 2 1 mol Pb. I 2 = 0. 39 mol KI mol 0. 39 mol KI = 0. 098 L of 4. 0 M KI = M 4. 0 M KI

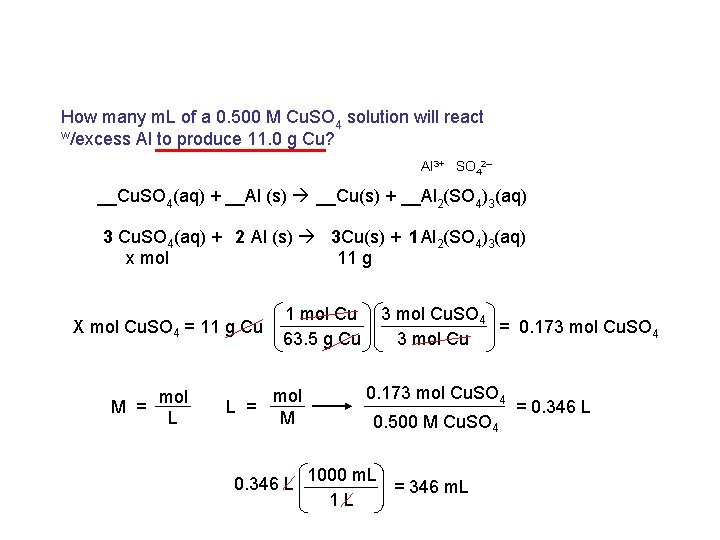
How many m. L of a 0. 500 M Cu. SO 4 solution will react w/excess Al to produce 11. 0 g Cu? Al 3+ SO 42– __Cu. SO 4(aq) + __Al (s) __Cu(s) + __Al 2(SO 4)3(aq) 3 Cu. SO 4(aq) + 2 Al (s) 3 Cu(s) + 1 Al 2(SO 4)3(aq) x mol 11 g X mol Cu. SO 4 = 11 g Cu mol M = L 1 mol Cu 63. 5 g Cu mol L = M 3 mol Cu. SO 4 = 0. 173 mol Cu. SO 4 3 mol Cu 0. 173 mol Cu. SO 4 0. 500 M Cu. SO 4 0. 346 L 1000 m. L = 346 m. L 1 L = 0. 346 L

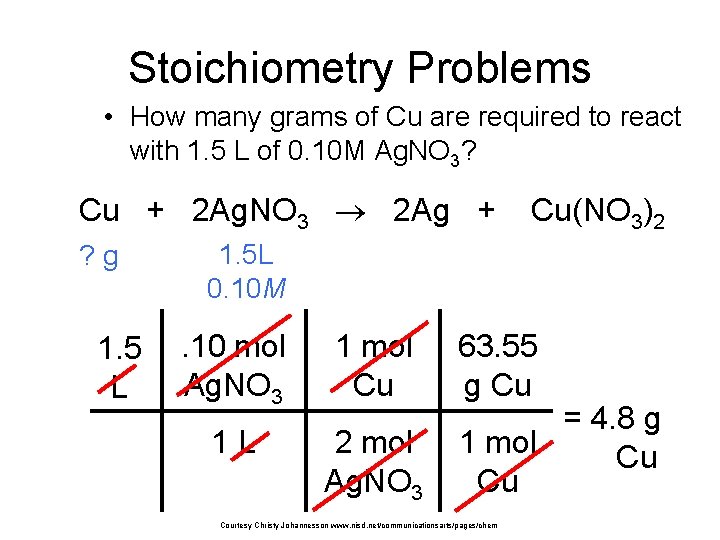
Stoichiometry Problems • How many grams of Cu are required to react with 1. 5 L of 0. 10 M Ag. NO 3? Cu + 2 Ag. NO 3 2 Ag + ? g 1. 5 L Cu(NO 3)2 1. 5 L 0. 10 M . 10 mol Ag. NO 3 1 mol Cu 1 L 2 mol Ag. NO 3 63. 55 g Cu = 4. 8 g 1 mol Cu Cu Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

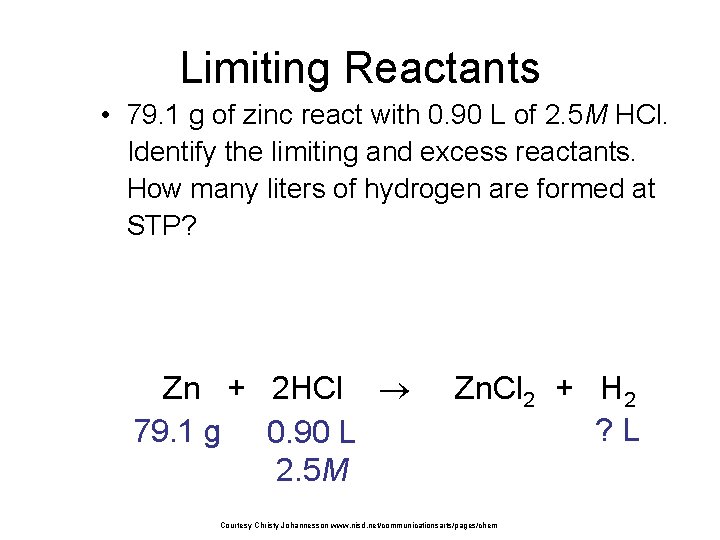
Limiting Reactants • 79. 1 g of zinc react with 0. 90 L of 2. 5 M HCl. Identify the limiting and excess reactants. How many liters of hydrogen are formed at STP? Zn + 2 HCl 79. 1 g 0. 90 L 2. 5 M Zn. Cl 2 + H 2 ? L Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

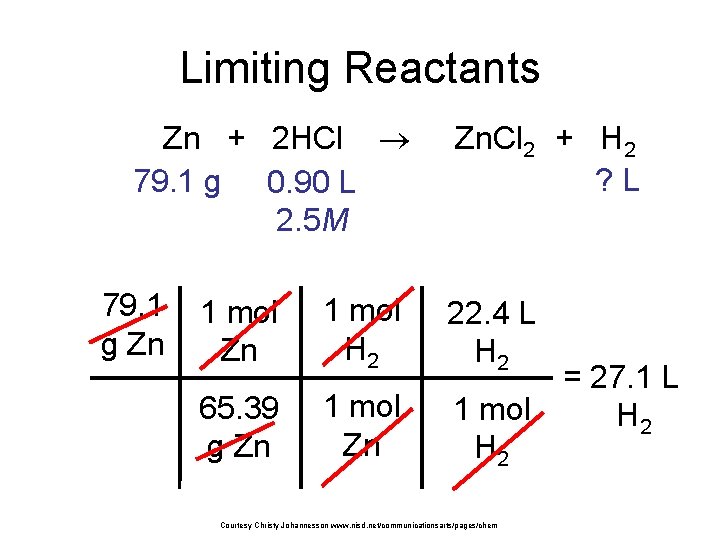
Limiting Reactants Zn + 2 HCl 79. 1 g 0. 90 L 2. 5 M 79. 1 g Zn Zn. Cl 2 + H 2 ? L 1 mol Zn 1 mol H 2 22. 4 L H 2 65. 39 g Zn 1 mol H 2 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem = 27. 1 L H 2

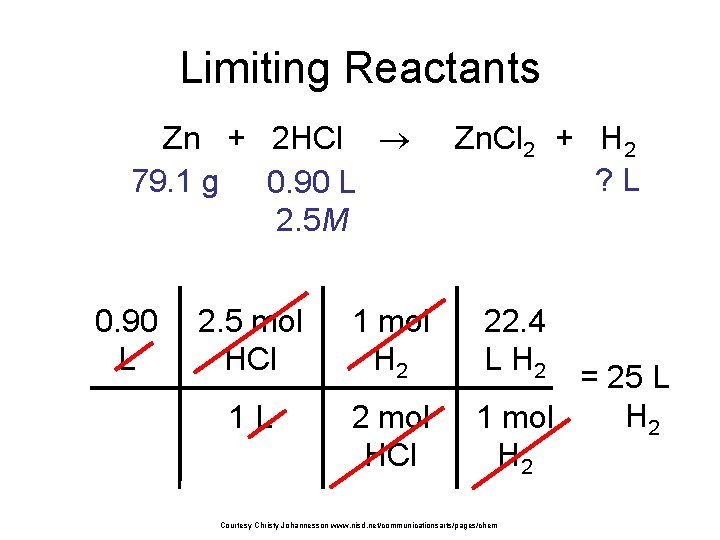
Limiting Reactants Zn + 2 HCl 79. 1 g 0. 90 L 2. 5 M 0. 90 L 2. 5 mol HCl 1 mol H 2 1 L 2 mol HCl Zn. Cl 2 + H 2 ? L 22. 4 L H 2 = 25 L H 2 1 mol H 2 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

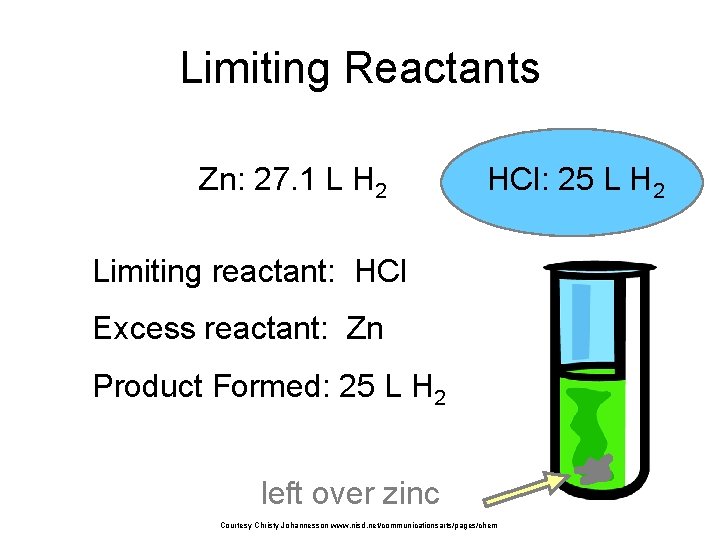
Limiting Reactants Zn: 27. 1 L H 2 HCl: 25 L H 2 Limiting reactant: HCl Excess reactant: Zn Product Formed: 25 L H 2 left over zinc Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem