MOLARITY A measurement of the concentration of a









- Slides: 10


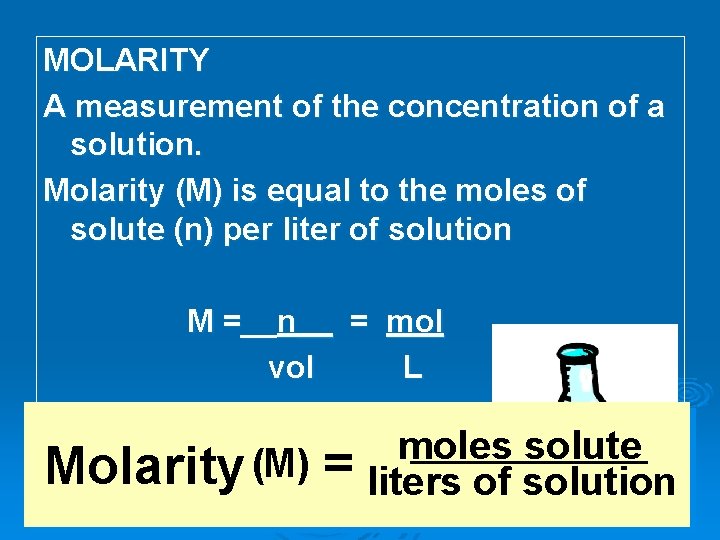
MOLARITY A measurement of the concentration of a solution. Molarity (M) is equal to the moles of solute (n) per liter of solution M =__n__ = mol vol L Molarity (M) = moles solute liters of solution

Suppose we had 1. 00 mole of sucrose (it's about 342. 3 grams) and proceeded to mix it into some water. It would dissolve and make sugar water. We keep adding water, dissolving and stirring until all the solid was gone. We then made sure that when everything was well-mixed, there was exactly 1. 00 liter of solution. What would be the molarity of this solution? A replacement for mol/L is often used. It is a capital M. So if you write 1. 00 M for the answer, then that is correct.

Example Calculate the molarity of a solution prepared by mixing 1. 5 g of Na. Cl in 500. 0 m. L of water. First calculate the moles of solute: 1. 5 g Na. Cl (1 mole Na. Cl) = 0. 0257 moles of Na. Cl 58. 45 g Na. Cl Next convert m. L to L: 0. 500 L Last, plug the appropriate values into the correct variables in the equation: M = n / V = 0. 0257 moles / 0. 500 L = 0. 051 mol/L

MOLARITY & DILUTION M 1 V 1 = M 2 V 2 The act of diluting a solution is to simply add more water (the solvent) thus leaving the amount of solute unchanged. The moles of solute after the dilution (na) are the same: n a = nb And the moles for any solution can be calculated by: n=MV A relationship can be established such that Ma V a = n b = M b V b

Calculate the molarity of a solution prepared by diluting 25. 0 m. L of 0. 05 M potassium iodide with 50. 0 m. L of water (the densities are similar). M 1 = 0. 05 mol/L V 1 = 25. 0 m. L M 2 = ? V 2 = 50. 0 + 25. 0 = 75. 0 m. L M 1 V 1 = M 2 V 2 M 1 V 1 = M 2 = (0. 05 mol/L) (25. 0 m. L) = 0. 0167 M V 2 75. 0 m. L of KI

MOLARITY & DILUTION Given a 6. 00 M HCl solution, how would you prepare 250. 0 m. L of 0. 150 M HCl? M 1 = 6. 00 mol/L M 2 = 0. 150 V 1 = ? m. L V 2 = 250. 0 m. L M 1 V 1 = M 2 V 2 = V 1 = (0. 150 mol/L) (250. 0 m. L) = 6. 25 m. L of 6 M HCl M 1 6. 00 mol/L

GROUP PROBLEMS 1)10. 0 g of acetic acid (CH 3 COOH) is dissolved in 500. 0 m. L of solution. What molarity results? 2) How many m. L of solution will result when 15. 0 g of H 2 SO 4 is dissolved to make a 0. 200 M solution? 3) Sea water contains roughly 28. 0 g of Na. Cl per liter. What is the molarity of sodium chloride in sea water? 4) What is the molarity of 245. 0 g of H 2 SO 4 dissolved in 1. 00 L of solution? 5) What is the molarity of 5. 30 g of Na 2 CO 3 dissolved in 400. 0 m. L solution?

GROUP PROBLEMS 6) What is the molarity of 5. 00 g of Na. OH in 750. 0 m. L of solution? 7) How many moles of Na 2 CO 3 are there in 10. 0 L of 2. 0 M solution? 8) How many moles of Na 2 CO 3 are in 10. 0 m. L of a 2. 0 M solution? 9) How many moles of Na. Cl are contained in 100. 0 m. L of a 0. 20 M solution? 10) What weight (in grams) of Na. Cl would be contained in problem 9? 11) What weight (in grams) of H 2 SO 4 would be needed to make 750. 0 m. L of 2. 00 M solution?

12) If I add 25 m. L of water to 125 m. L of a 0. 15 M Na. OH solution, what will the molarity of the diluted solution be? 13) If I add water to 100 m. L of a 0. 15 M Na. OH solution until the final volume is 150 m. L, what will the molarity of the diluted solution be? 14) How much 0. 05 M HCl solution can be made by diluting 250 m. L of 10 M HCl? 15) I have 345 m. L of a 1. 5 M Na. Cl solution. If I boil the water until the volume of the solution is 250 m. L, what will the molarity of the solution be? 16) How much water would I need to add to 500 m. L of a 2. 4 M KCl solution to make a 1. 0 M solution?