Molar Volume 1 mol of a gas STP

- Slides: 21

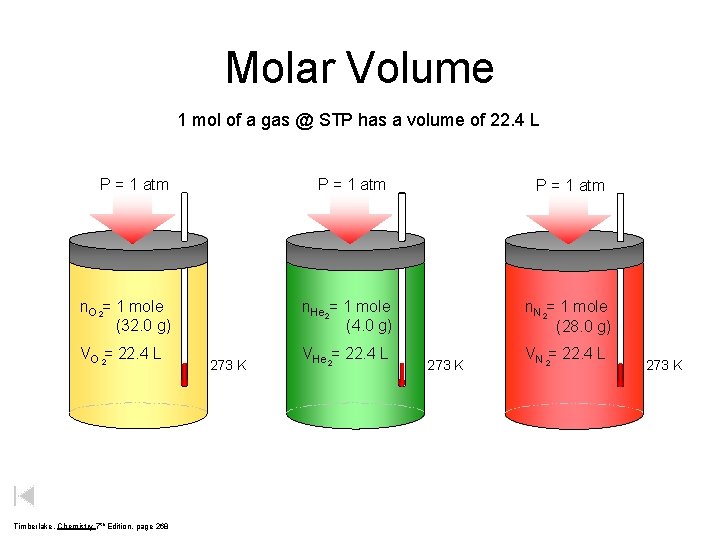
Molar Volume 1 mol of a gas @ STP has a volume of 22. 4 L P = 1 atm n. O 2= 1 mole (32. 0 g) VO 2= 22. 4 L Timberlake, Chemistry 7 th Edition, page 268 273 K P = 1 atm n. He 2= 1 mole (4. 0 g) n. N 2= 1 mole (28. 0 g) VHe 2= 22. 4 L 273 K VN 2= 22. 4 L 273 K

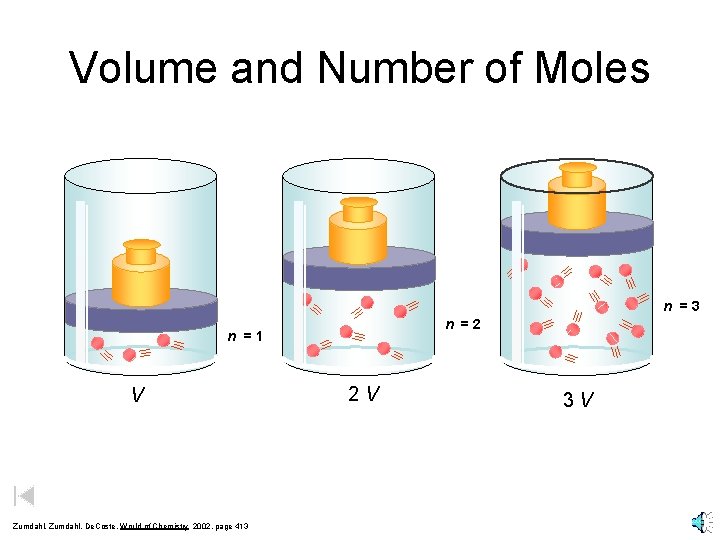
Volume and Number of Moles n = 3 n = 2 n = 1 V Zumdahl, De. Coste, World of Chemistry 2002, page 413 2 V 3 V

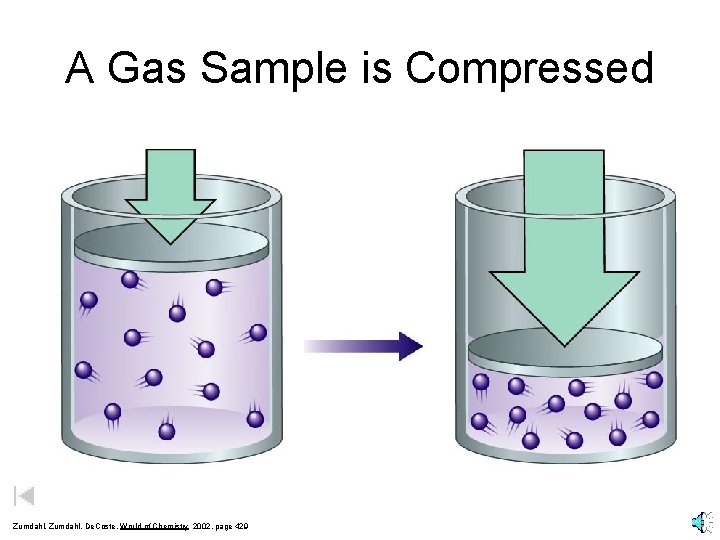
A Gas Sample is Compressed Zumdahl, De. Coste, World of Chemistry 2002, page 429

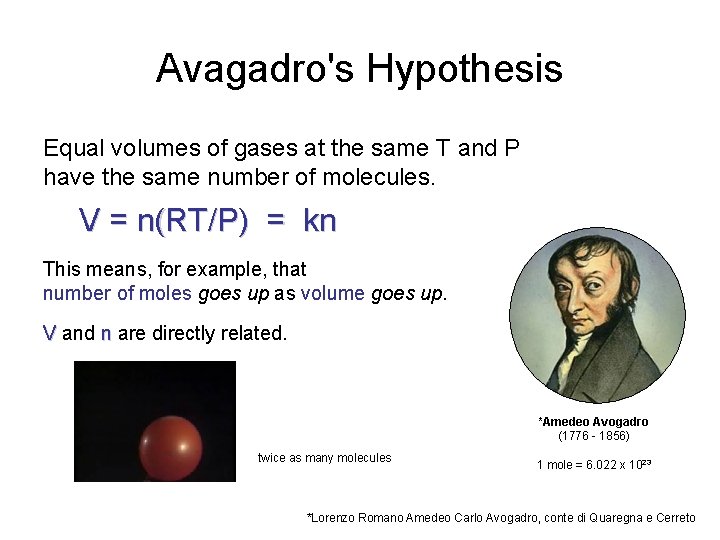
Avagadro's Hypothesis Equal volumes of gases at the same T and P have the same number of molecules. V = n(RT/P) = kn This means, for example, that number of moles goes up as volume goes up. V and n are directly related. *Amedeo Avogadro (1776 - 1856) twice as many molecules 1 mole = 6. 022 x 1023 *Lorenzo Romano Amedeo Carlo Avogadro, conte di Quaregna e Cerreto

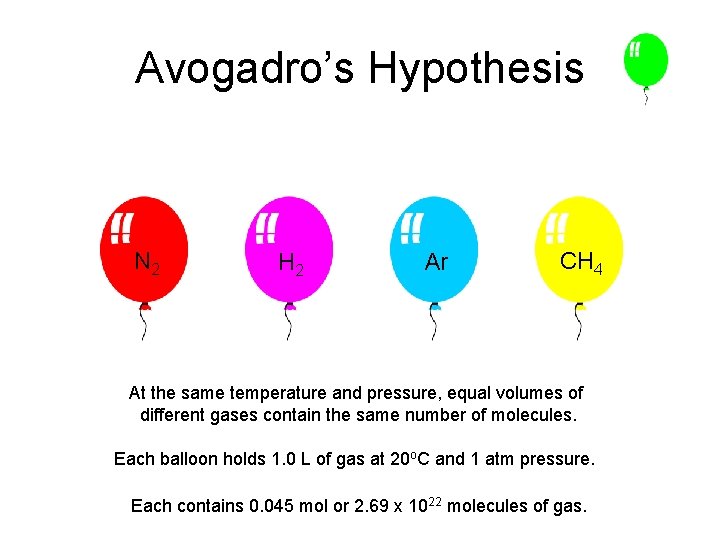
Avogadro’s Hypothesis N 2 H 2 Ar CH 4 At the same temperature and pressure, equal volumes of different gases contain the same number of molecules. Each balloon holds 1. 0 L of gas at 20 o. C and 1 atm pressure. Each contains 0. 045 mol or 2. 69 x 1022 molecules of gas.

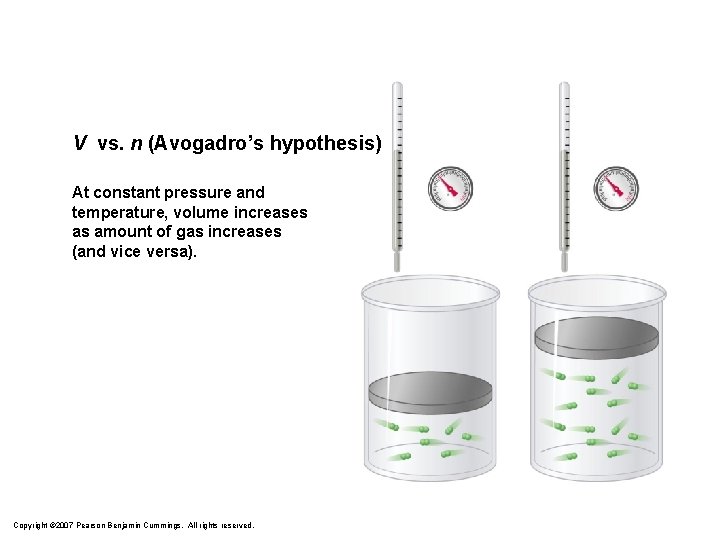
V vs. n (Avogadro’s hypothesis) At constant pressure and temperature, volume increases as amount of gas increases (and vice versa). Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.

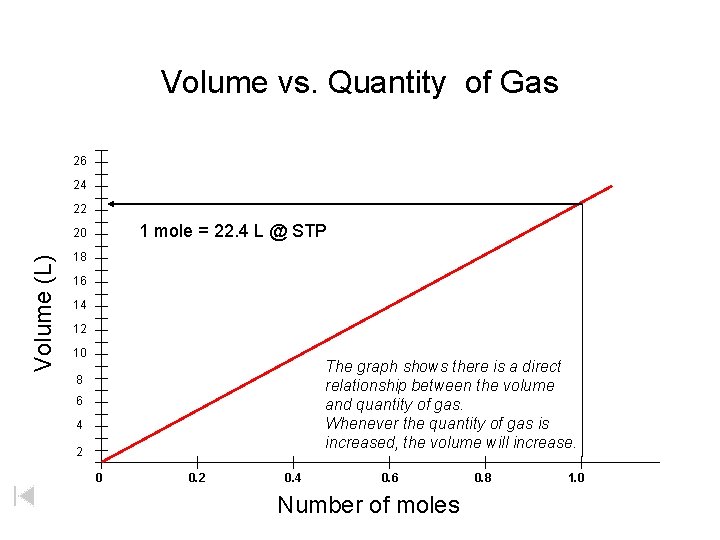
Volume vs. Quantity of Gas 26 24 22 Volume (L) 20 1 mole = 22. 4 L @ STP 18 16 14 12 10 8 6 4 2 The graph shows there is a direct relationship between the volume and quantity of gas. Whenever the quantity of gas is increased, the volume will increase. 0 0. 2 0. 4 0. 6 0. 8 1. 0 Number of moles

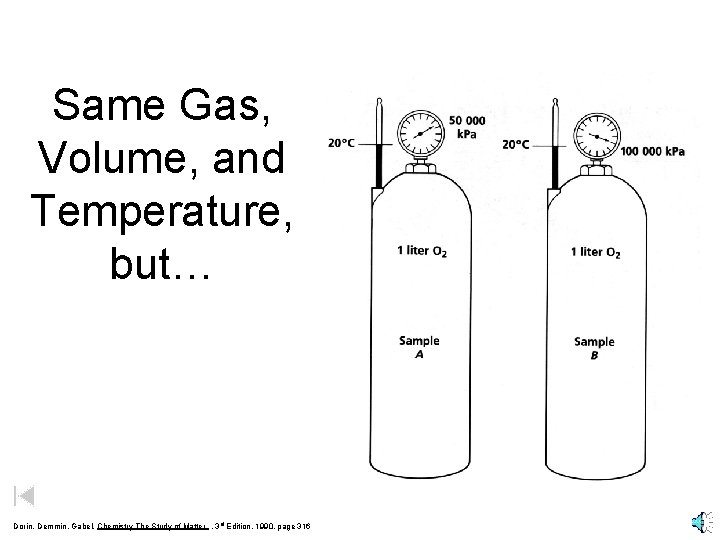
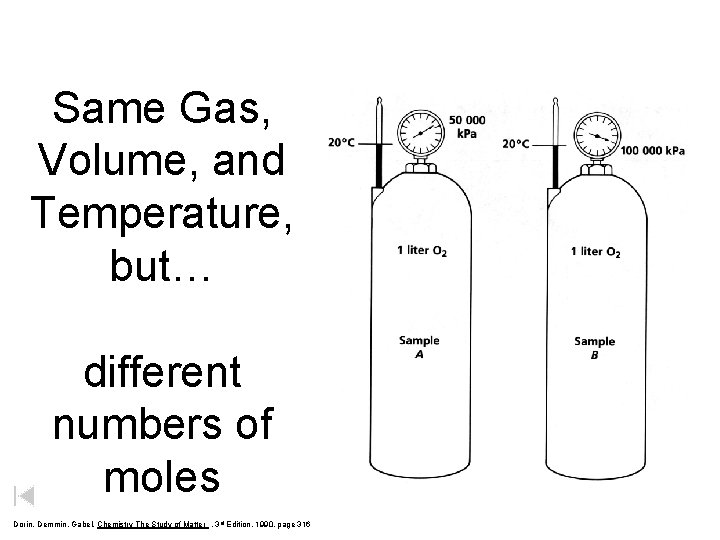
Same Gas, Volume, and Temperature, but… Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 316

Same Gas, Volume, and Temperature, but… different numbers of moles Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 316

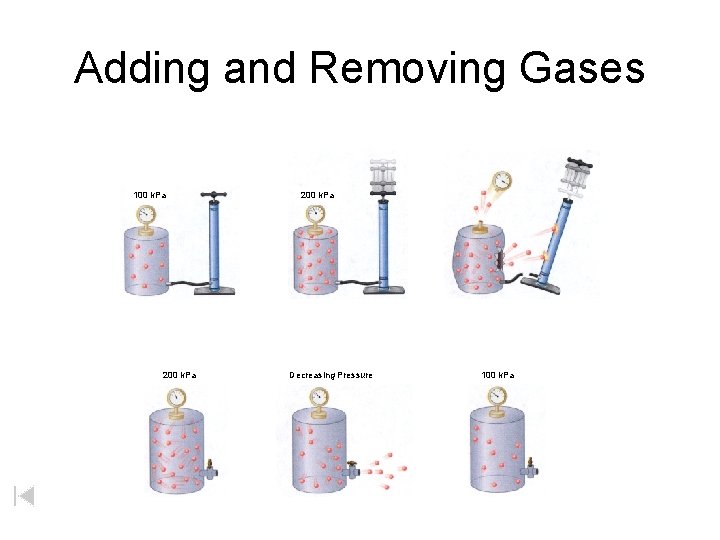
Adding and Removing Gases 100 k. Pa 200 k. Pa Decreasing Pressure 100 k. Pa

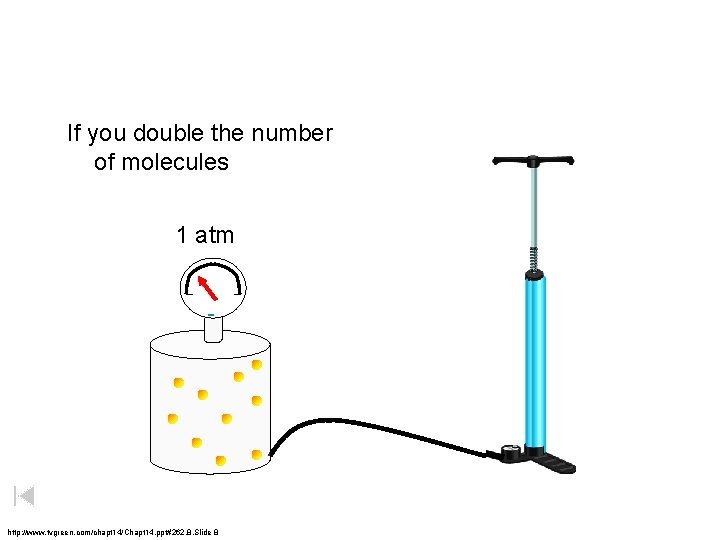
If you double the number of molecules 1 atm http: //www. tvgreen. com/chapt 14/Chapt 14. ppt#262, 8, Slide 8

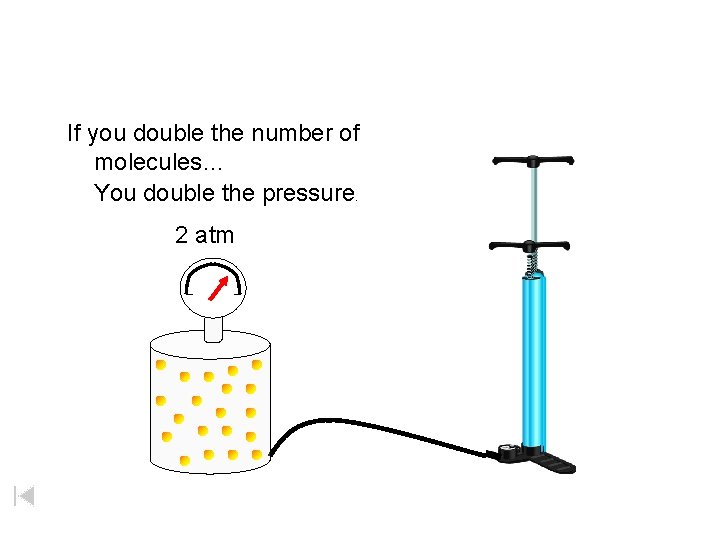
If you double the number of molecules… You double the pressure. 2 atm

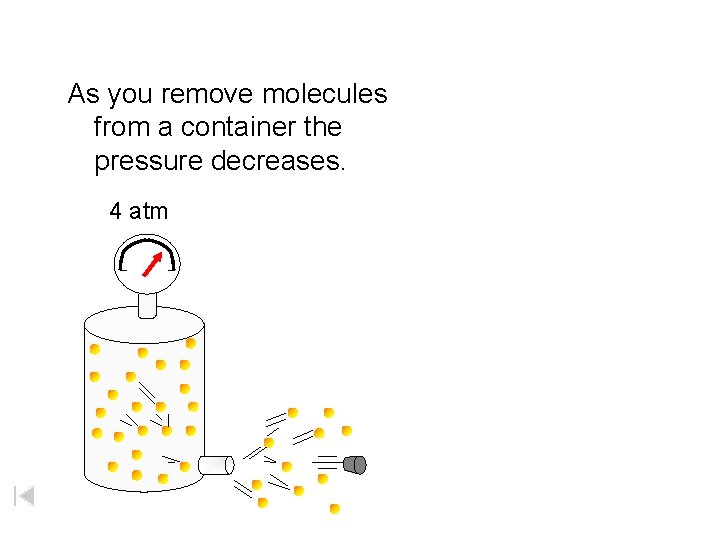
As you remove molecules from a container the pressure decreases. 4 atm

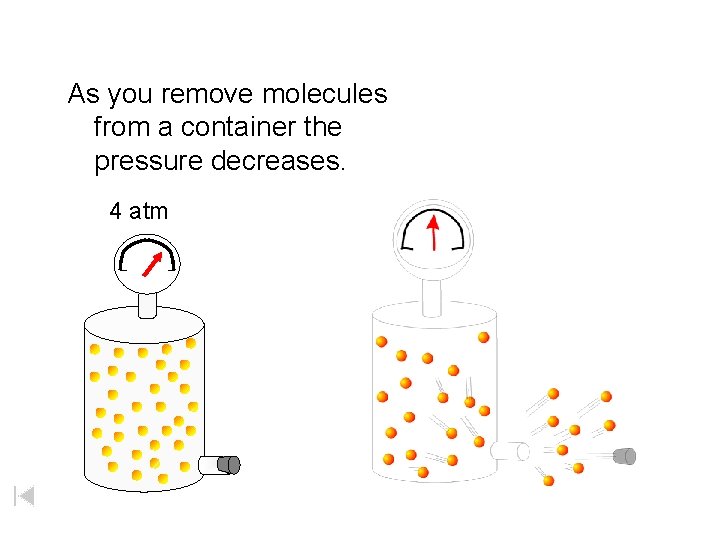
As you remove molecules from a container the pressure decreases. 4 atm

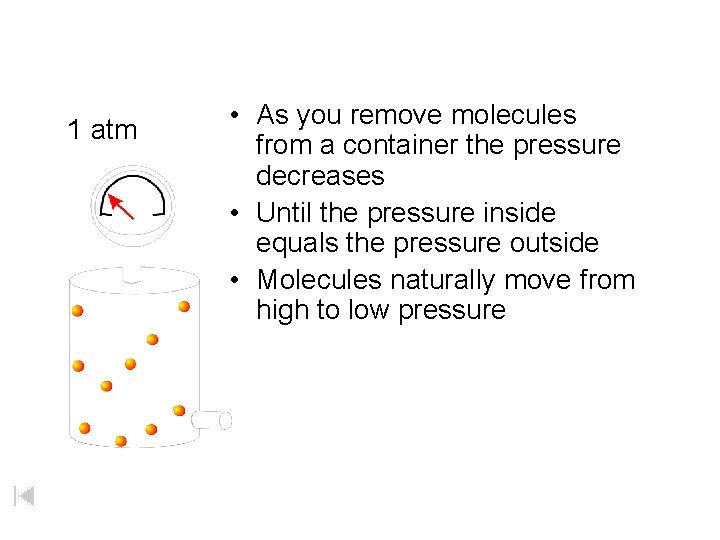
1 atm • As you remove molecules from a container the pressure decreases • Until the pressure inside equals the pressure outside • Molecules naturally move from high to low pressure

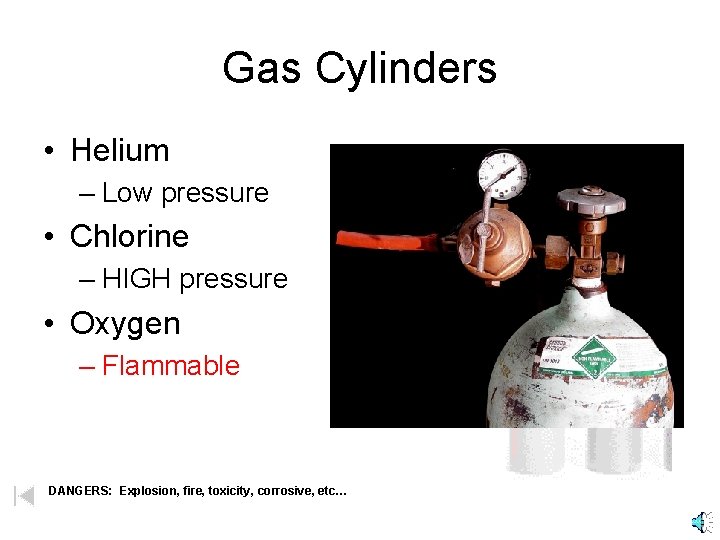
Gas Cylinders • Helium – Low pressure • Chlorine – HIGH pressure • Oxygen – Flammable DANGERS: Explosion, fire, toxicity, corrosive, etc…

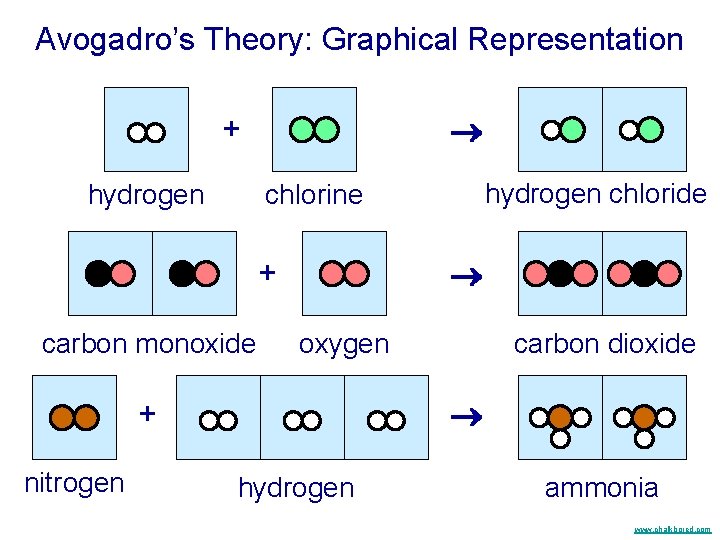
Avogadro’s Theory: Graphical Representation + hydrogen + carbon monoxide oxygen carbon dioxide + nitrogen hydrogen chloride chlorine hydrogen ammonia www. chalkbored. com

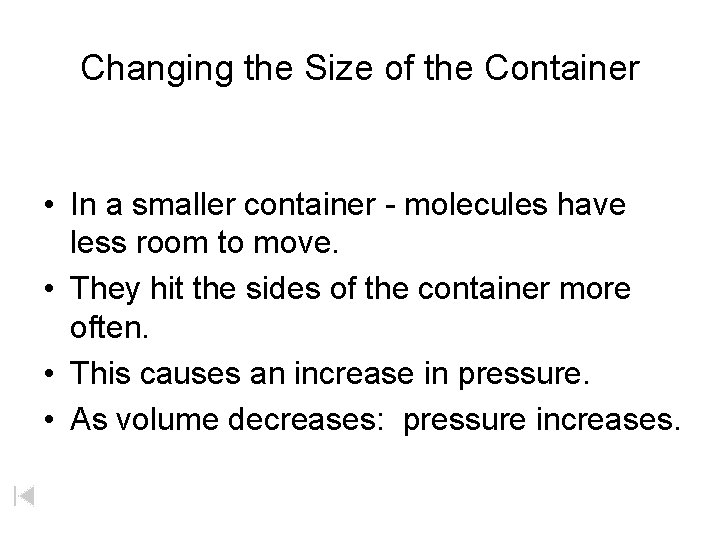
Changing the Size of the Container • In a smaller container - molecules have less room to move. • They hit the sides of the container more often. • This causes an increase in pressure. • As volume decreases: pressure increases.

Theory “works, ” except at high pressures and low temps **Two gases w/same # of particles and at same temp. and pressure have the same kinetic energy. KE is related to mass and velocity (KE = ½ m v 2) To keep same KE, as m , v must OR

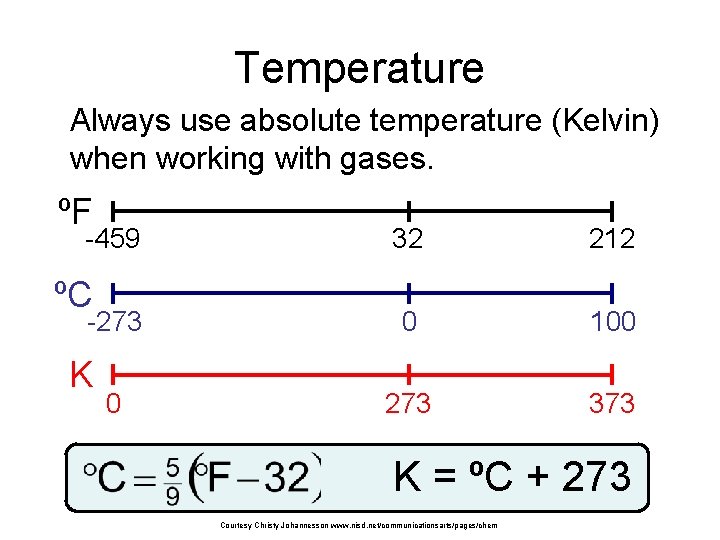
Temperature Always use absolute temperature (Kelvin) when working with gases. ºF -459 ºC -273 K 0 32 212 0 100 273 373 K = ºC + 273 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

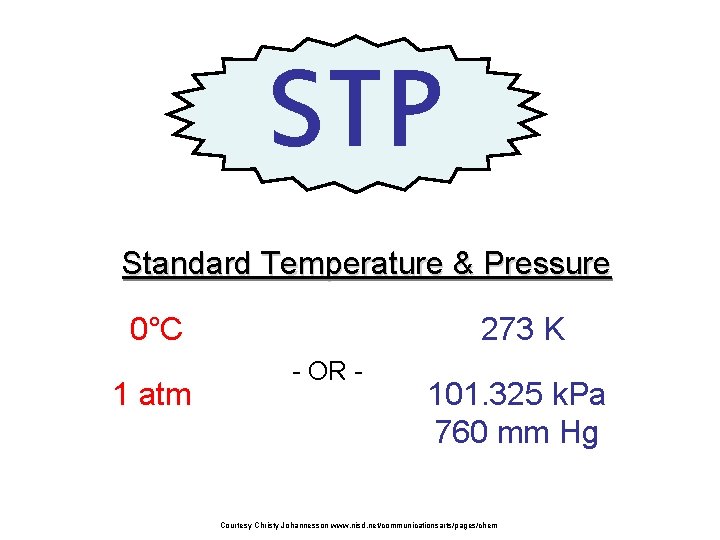
STP Standard Temperature & Pressure 273 K 0°C 1 atm - OR - 101. 325 k. Pa 760 mm Hg Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem