Molar Mass M Topic 1 2 Mass relative









- Slides: 17

Molar Mass (M) Topic 1. 2

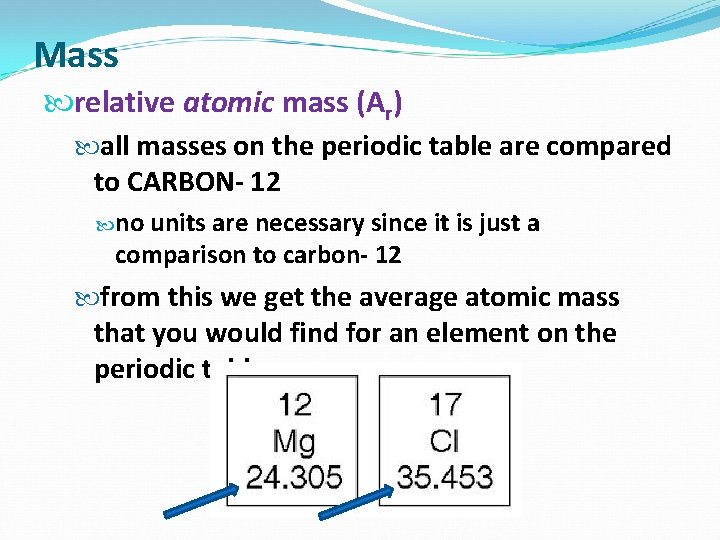
Mass relative atomic mass (Ar) all masses on the periodic table are compared to CARBON- 12 no units are necessary since it is just a comparison to carbon- 12 from this we get the average atomic mass that you would find for an element on the periodic table

relative molecular mass (Mr) simply comparing a given molecule to carbon CO 2 is 44. C 60 is 720, O 2 is 16(2) = 32 atomic or molecular mass this is simply the mass of one atom or molecule using a very small unit of measurement called a. m. u. therefore CO 2 is 44 a. m. u. molar mass (M) this is how many grams you would have in a mole of a substance therefore CO 2 is 44 g/mol

molar mass continued… • simply change the relative atomic mass from the periodic table for the element/molecule/ compound to grams • • oxygen’s atomic mass is 16. 00 amu therefore 1 mole of oxygen has a molar mass is 16. 00 g/mol sugar’s (C 6 H 12 O 6) atomic mass is 180. 0 amu • carbon: 12. 00(6) = 72. 00 • hydrogen: 1. 00(12) = 12. 00 • oxygen: 16. 00(6) = 96. 00 • therefore 1 mole of sugar has a molar mass of 180. 00 g/mol

Calculate the Molar Mass of Na 3 PO 4. Atom Na P O # 3 1 4 Atomic Mass X 23. 0 X 31. 0 X 16. 0 Total = = Total 69. 0 31. 0 64. 0 164. 0 Therefore the molar mass is 164. 0 g mol-1

• Give the molar mass (mass of one mole): • Fe → 55. 9 g/mol • C 2 H 6 → 30. 1 g/mol • Fe(OH)2 → 89. 9 g/mol • C 12 H 22 O 11 → 342. 3 g/mol • Cu. SO 4 · 5 H 2 O → 238 g/mol

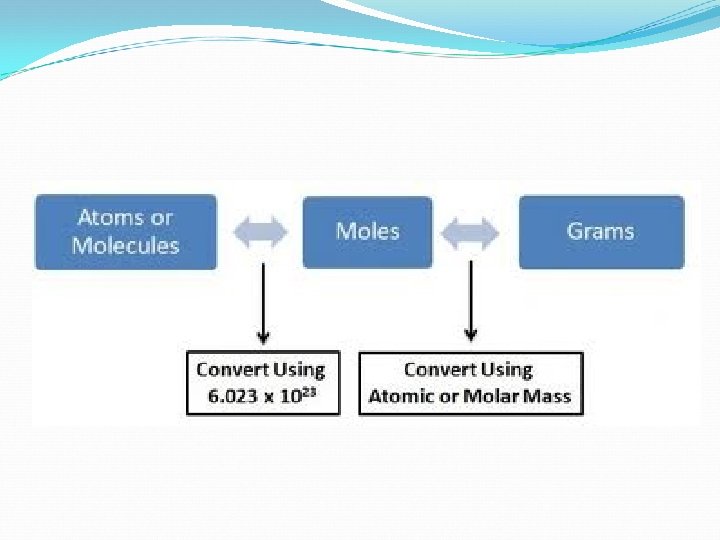
Converting Moles to Grams and Grams to Moles conversion factor will be: 1 mole molar mass OR molar mass__ 1 mole


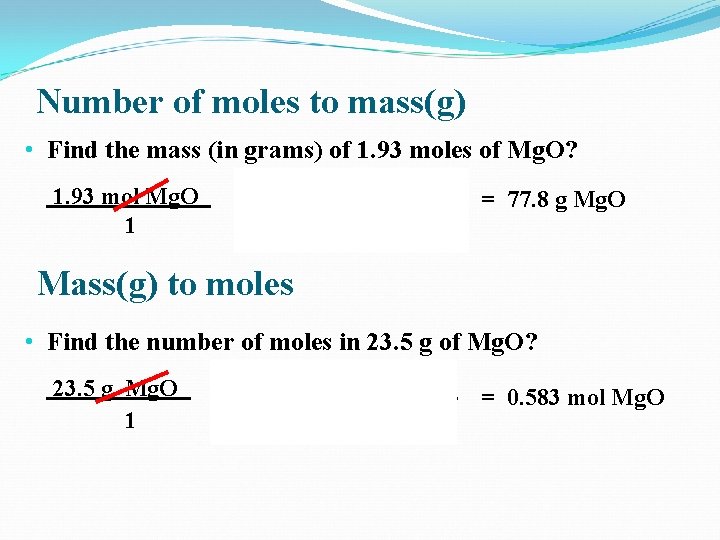
Number of moles to mass(g) • Find the mass (in grams) of 1. 93 moles of Mg. O? 1. 93 mol Mg. O 1 x 40. 3 g Mg. O 1 mol Mg. O = 77. 8 g Mg. O Mass(g) to moles • Find the number of moles in 23. 5 g of Mg. O? 23. 5 g Mg. O 1 x 1 mole Mg. O 40. 3 g Mg. O = 0. 583 mol Mg. O

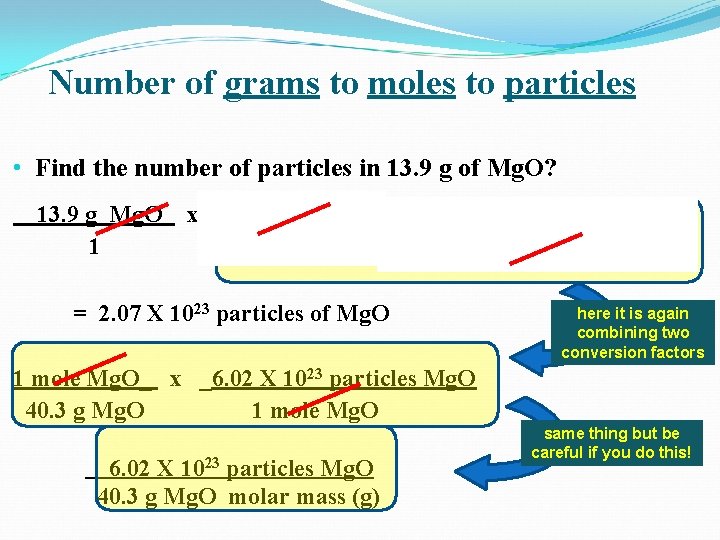
Number of grams to moles to particles • Find the number of particles in 13. 9 g of Mg. O? 13. 9 g Mg. O 1 x 1 mole Mg. O_ x _6. 02 X 1023 particles Mg. O 40. 3 g Mg. O 1 mole Mg. O = 2. 07 X 1023 particles of Mg. O here it is again combining two conversion factors 1 mole Mg. O_ x _6. 02 X 1023 particles Mg. O 40. 3 g Mg. O 1 mole Mg. O 6. 02 X 1023 particles Mg. O 40. 3 g Mg. O molar mass (g) same thing but be careful if you do this!

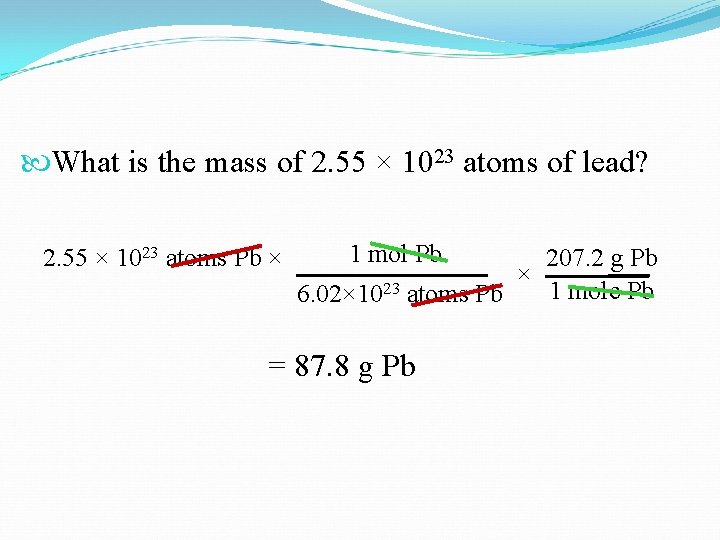
What is the mass of 2. 55 × 1023 atoms of lead? 2. 55 × 1023 atoms Pb × 1 mol Pb 207. 2 g Pb × 23 1 mole Pb 6. 02× 10 atoms Pb = 87. 8 g Pb

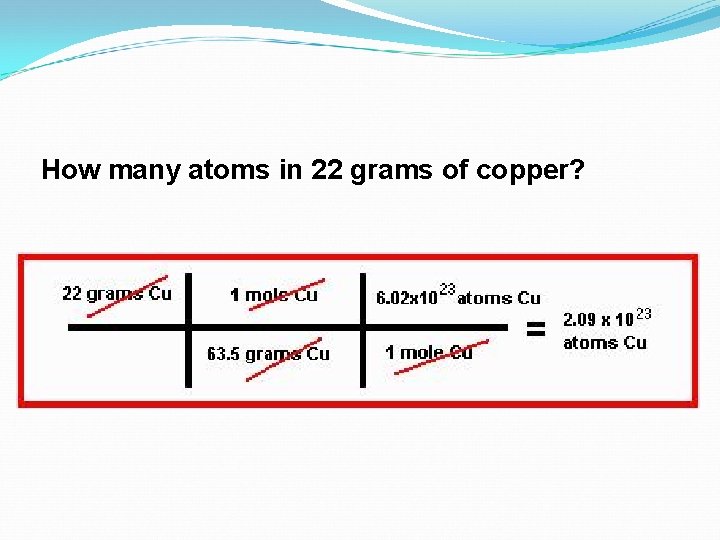
How many atoms in 22 grams of copper?

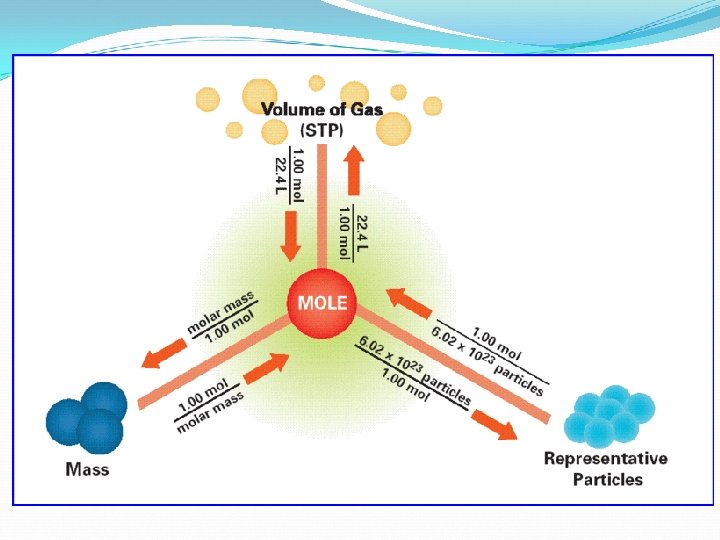
The Mole Road Map Mass mole g g mole “Stuff” 6. 022 x 1023 mole Mole mole. 22. 7 L mole Stuff: mole. 6. 022 x 1023 • Atoms • Molecules • Particles • Electrons • Ions • Formula Units For Gases only: @ STP (Standard Temp & Pressure) (0 o. C (273 K) & 1 atm) Volume



