MOLAR CONVERSIONS ATOMS TO MOLES MOLES TO ATOMS













- Slides: 15

MOLAR CONVERSIONS ATOMS TO MOLES, MOLES TO ATOMS, MASS TO ATOMS, ATOMS TO MASS, MASS TO MASS 1

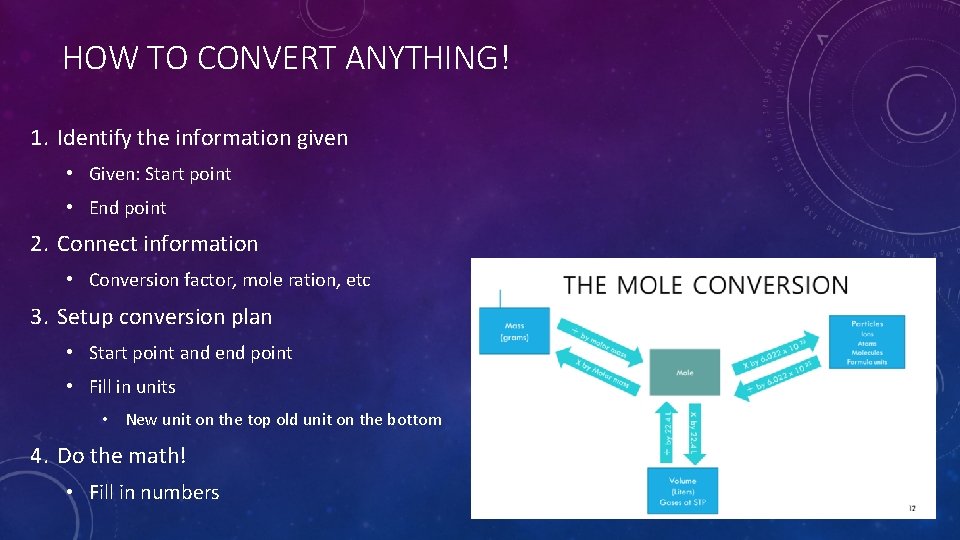
HOW TO CONVERT ANYTHING! 1. Identify the information given • Given: Start point • End point 2. Connect information • Conversion factor, mole ration, etc 3. Setup conversion plan • Start point and end point • Fill in units • New unit on the top old unit on the bottom 4. Do the math! • Fill in numbers 2

ATOMS TO MOLE, MOLES TO ATOMS • 3

LETS CONVERT ! Atoms to moles • 4

KEEP CONVERTING! • 5

MOLES TO MASS/ MASS TO MOLES • SAME PROCESS AS PARTICLES TO MOLES/MOLE TO PARTICLES • Just different units! • A few things to remember • 1 moles = molar mass • Elements: average atomic mass (periodic table) • Compounds: Must calculate 6

LETS CONVERT! • • 7

MOLES TO VOLUME/VOLUME TO MOLES • SAME PROCESS AS PARTICLES TO MOLES/MOLE TO PARTICLES • Just different units! • A few things to remember • 1 moles = 22. 4 L @STP • S: standard • T: temperature ( 0 o. C) • P: Pressure (1 atm = 101. 325 k. Pa = 760 mm Hg) 8

CONVERSION TIME • • 9

MASS TO PARTICLES : OF THE SAME COMPOUND A few things to remember 1. 6. 022 x 10 23 particles = 1 mole of ANY compound • Particles can be: ions, atoms, formula unit, or molecules 2. 1 moles = molar mass • Elements: average atomic mass (periodic table) • Compounds: Must calculate Cannot complete in one step! Grams and particles cannot connect in one step. Must go to Moles 1 st 10

TIME TO CONVERT Side Calculation N: (2) 14. 007= 28. 014 g/mol • 11

MASS OF ONE COMPOUND TO MASS OF ANOTHER • Five Steps 1. Label what is given 1. Start point/ endpoint 2. Connect the information 1. Mole ratio (from balanced equation) 3. Setup first conversion 4. Setup second conversion 5. Setup third conversion and solve 12

STEPS 1 AND 2 How many grams of potassium chloride, KCl, are produced if 25. 0 g of potassium chlorate, KCl. O 3, decompose? 25. 0 g ? g 2 KCl. O 3 → 2 KCl + 3 O 2 2 moles KCl. O 3 : 2 mole KCl 13

STEP 3 AND 4 • • Side Calculation K: 39. 089 g/mol Cl: 35. 454 g/mol O: (3) 15. 999 = 47. 997 g/mol 122. 549 g/mol 14

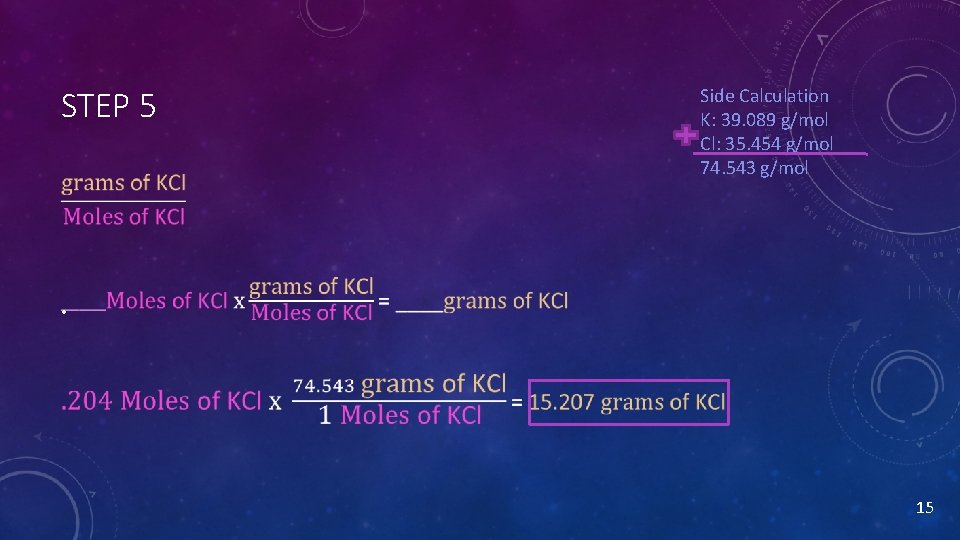
STEP 5 Side Calculation K: 39. 089 g/mol Cl: 35. 454 g/mol 74. 543 g/mol • 15