MODULE A 4 MEASUREMENT SYSTEMS SCIENTIFIC NOTATION OBJECTIVES
MODULE A - 4 MEASUREMENT SYSTEMS & SCIENTIFIC NOTATION
OBJECTIVES • At the end of this module, the student will be able to… Ø Identify and compare the systems of measurement used in the clinical setting. Ø Identify the standard prefixes used in the metric system Ø State the metric units of length, mass, volume, time, and temperature. Ø Distinguish between the metric units for liquid (m. L) and solid volume (cc) measurements.
Measurement systems • Method of quantifying matter Ø Solids, liquids & gases • Quantities include: Ø Length Ø Area Ø Weight Ø Volume Ø Pressure Ø Temperature Ø Time • Systems used in medicine: A. Conventional B. Metric C. Standard International
Conventional Systems • Also known as: Ø British Ø English Ø U. S Customary Ø (FPS) foot, pound, second • Commonly used in U. S. FPS

Examples of length & area 12 inches = 1 foot 3 feet (36 inches) = 1 yard 220 yards = 1 furlong 8 furlongs = 1 mile 1, 760 yards = 1 mile 5, 280 feet = 1 mile 1 sq. foot (foot 2) = 122 sq. inches 1 sq. yard (yard 2) = 9 sq. feet 43, 560 sq. feet = 1 acre 1 sq. mile (mile 2) = 640 acres
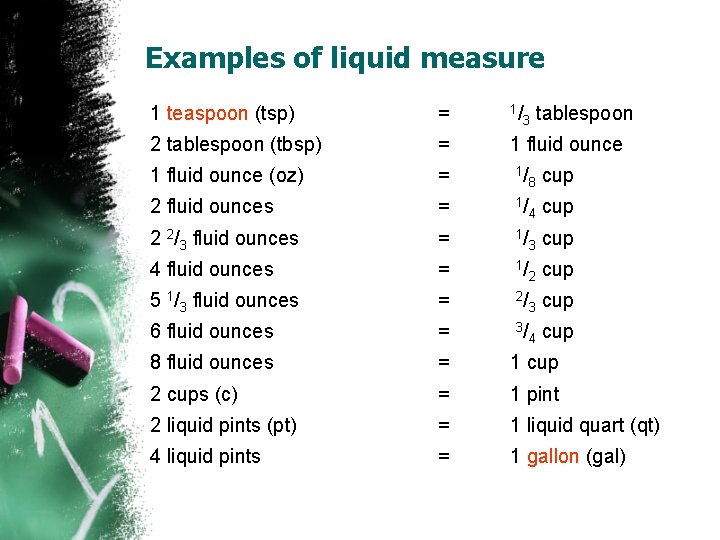
Examples of liquid measure 1 teaspoon (tsp) = 1/ 2 tablespoon (tbsp) = 1 fluid ounce (oz) = 1/ 8 cup 2 fluid ounces = 1/ 4 cup 2 2/3 fluid ounces = 1/ 3 cup 4 fluid ounces = 1/ 2 cup 5 1/3 fluid ounces = 2/ 3 cup 6 fluid ounces = 3/ 4 cup 8 fluid ounces = 1 cup 2 cups (c) = 1 pint 2 liquid pints (pt) = 1 liquid quart (qt) 4 liquid pints = 1 gallon (gal) 3 tablespoon

Examples of dry measure 1 dry quart = 2 dry pints 8 dry pints = 1 peck 4 pecks = 1 bushel
Standard International (SI) • Simplified modification of metric system. • Worldwide effort started in 1960 s to standardize to this system. • Also known as: Ø (MKS) meter, kilogram, second MKS
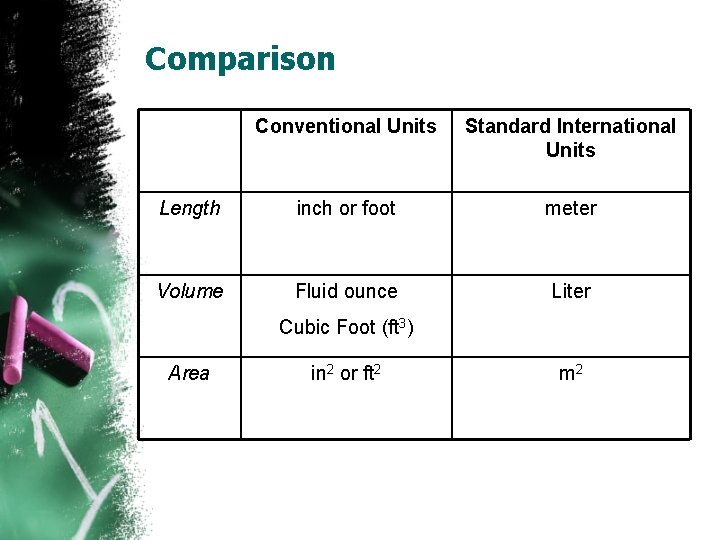
Comparison Conventional Units Standard International Units Length inch or foot meter Volume Fluid ounce Liter Cubic Foot (ft 3) Area in 2 or ft 2 m 2
Metric System • Developed in Europe. • Has all units based on multiples of 10. • Also known as: Ø (CGS) centimeter, gram, second CGS
Measurements in Respiratory Therapy • Length Ø Meter (m) • Volume Ø Liter (L) • Mass Ø Gram (g) • Time Ø Seconds (sec) • Temperature Ø Centigrade (Celsius), Kelvin, Fahrenheit • Pressure Ø Centimeters of Water (cm H 2 O), Pounds per square inch (psi), Millimeters of mercury (mm Hg), Torr, Pascal (Pa), and Atmospheres (atm) • Force Ø Dynes

Conversion • Conversion within the metric system is easy Ø Everything based on multiples of ten. • Conversion from one system to the other: Ø Must know the conversion factors.

Conversion • Conversion within these systems or from one system to the other: Ø You Must know how to do metric conversions. Ø I will provide the S. I. and conventional factors on an exam or quiz. • There are too many to memorize. • Gimli Glider & Mars Climate Orbiter
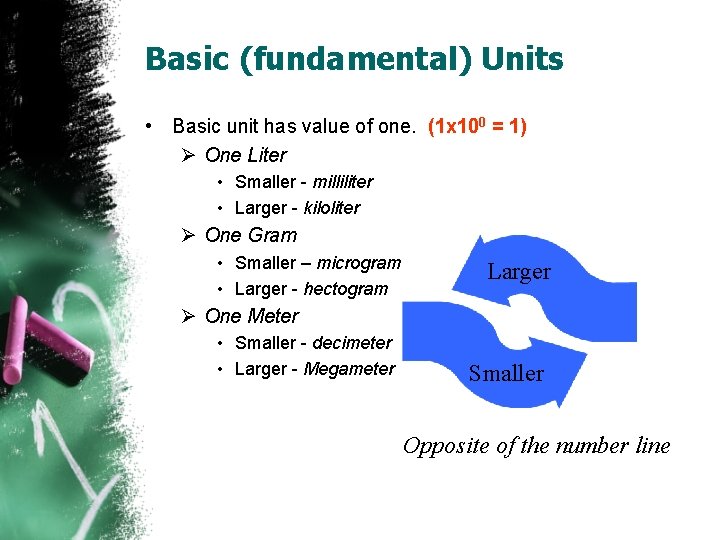
Basic (fundamental) Units • Basic unit has value of one. (1 x 100 = 1) Ø One Liter • Smaller - milliliter • Larger - kiloliter Ø One Gram • Smaller – microgram • Larger - hectogram Larger Ø One Meter • Smaller - decimeter • Larger - Megameter Smaller Opposite of the number line
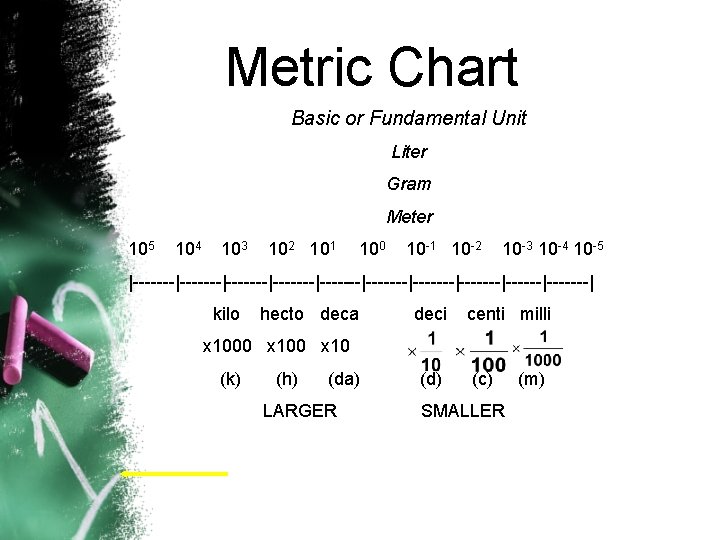
Metric Chart Basic or Fundamental Unit Liter Gram Meter 105 104 103 102 101 100 10 -1 10 -2 10 -3 10 -4 10 -5 |-------|-------|-------|-------|-------| kilo hecto deca deci centi milli x 1000 x 10 (k) (h) (da) LARGER (d) (c) SMALLER (m)

Greek Prefixes - Units to the left of the basic unit and larger. • BASIC UNIT = One Liter, Gram or Meter • • • 10 1 10 2 10 3 10 4 10 5 10 6 10 7 10 8 10 9 deca (da) hecto (h) kilo (k) 10 x larger 1000 x larger 10 1000 Mega (M) 1, 000 x 1, 000 Giga (G) 1, 000, 000 x 1, 000, 000

Latin Prefixes Units to the right of the basic unit and smaller. • BASIC UNIT = One Liter, Gram or Meter • 10 -1 deci (d); 10 x smaller; 1/10; x 0. 1 • 10 -2 centi (c); 100 x smaller; 1/100; x 0. 01 • 10 -3 milli (m); 1000 x smaller; 1/1, 000; x 0. 001 • 10 -4 • 10 -5 • 10 -6 micro (m) or (mc); 1, 000 x smaller; 1/1, 000; x 0. 000001 • 10 -7 • 10 -8 • 10 -9 nano (n); 1, 000, 000 x smaller; 1/1, 000, 000; x 0. 00001 • 10 -10 Angstrom (Å); 10, 000, 000 x smaller; 1/10, 000, 000; x 0. 000001
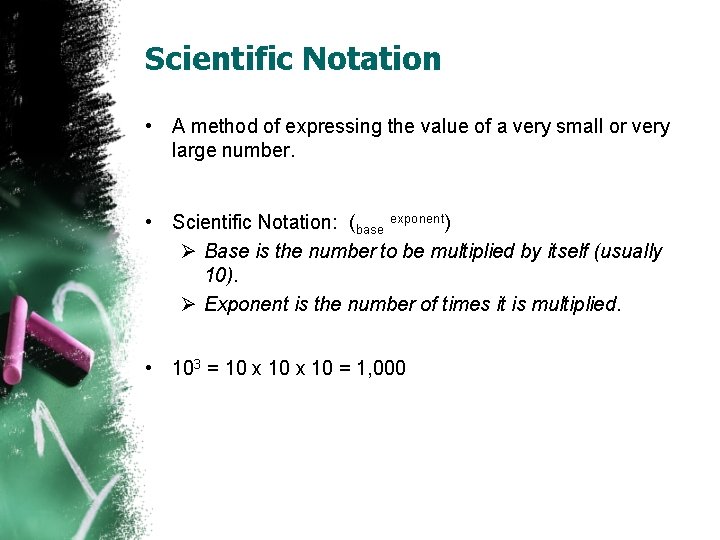
Scientific Notation • A method of expressing the value of a very small or very large number. • Scientific Notation: (base exponent) Ø Base is the number to be multiplied by itself (usually 10). Ø Exponent is the number of times it is multiplied. • 103 = 10 x 10 = 1, 000

Scientific Notation Ø Example: • A kilometer is 1, 000 times larger than a meter • Count the zeros (that equals exponent) • 103 • 10 x 10 times larger
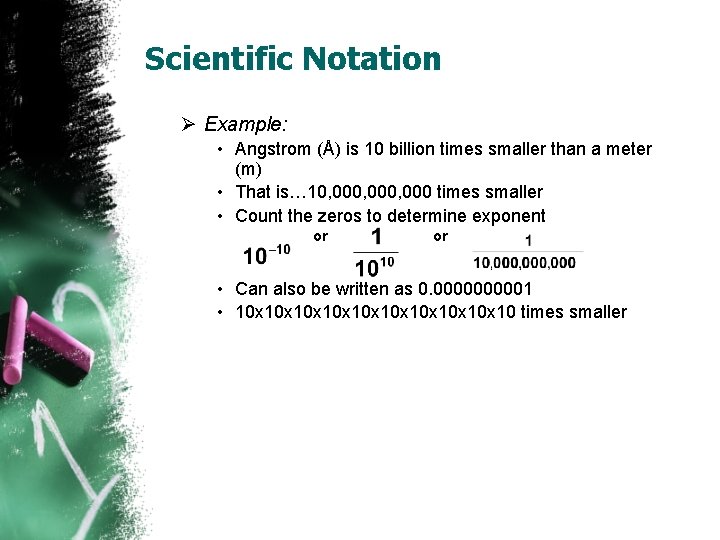
Scientific Notation Ø Example: • Angstrom (Å) is 10 billion times smaller than a meter (m) • That is… 10, 000, 000 times smaller • Count the zeros to determine exponent or or • Can also be written as 0. 000001 • 10 x 10 x 10 x 10 times smaller
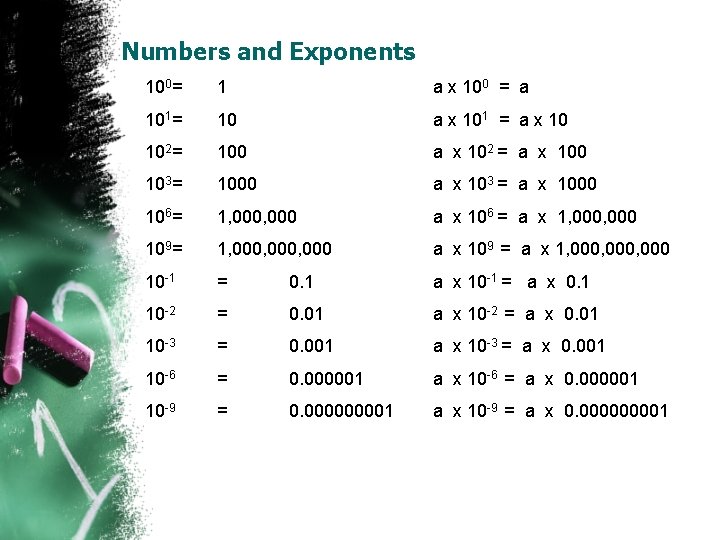
Numbers and Exponents 100= 1 a x 100 = a 101= 10 a x 101 = a x 10 102= 100 a x 102 = a x 100 103= 1000 a x 103 = a x 1000 106= 1, 000 a x 106 = a x 1, 000 109= 1, 000, 000 a x 109 = a x 1, 000, 000 10 -1 = 0. 1 a x 10 -1 = a x 0. 1 10 -2 = 0. 01 a x 10 -2 = a x 0. 01 10 -3 = 0. 001 a x 10 -3 = a x 0. 001 10 -6 = 0. 000001 a x 10 -6 = a x 0. 000001 10 -9 = 0. 00001 a x 10 -9 = a x 0. 00001

Numbers and Exponents Positive exponent = # of zeros 5 x 100 = 5 5 x 101 = 50 5 x 102 = 500 5 x 103 = 5000 5 x 106 = 5, 000 5 x 109 = 5, 000, 000 Negative exponent = # of decimal places 5 x 10 -1 = 0. 5 5 x 10 -2 = 0. 05 5 x 10 -3 = 0. 005 5 x 10 -6 = 0. 000005 5 x 10 -9 = 0. 00005

Examples - Avogadro’s Number Expresses the number of atoms in one mole of a gas Long form: 602, 000, 000, 000 atoms Scientific notation: 6. 02 x 10 23 atoms Process: Count over to the left, the number of decimal places to get a number between 1 & 10
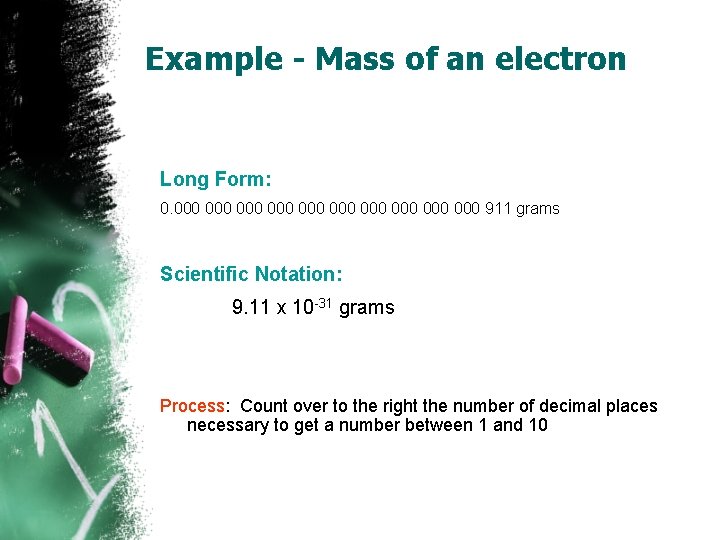
Example - Mass of an electron Long Form: 0. 000 000 000 911 grams Scientific Notation: 9. 11 x 10 -31 grams Process: Count over to the right the number of decimal places necessary to get a number between 1 and 10

Practice: Express the following exponentially • 500 = 5 x 102 (count over to left 2 decimal places) • 93, 000 = _________ • 0. 0003 = _________ • 0. 000000024 = _________
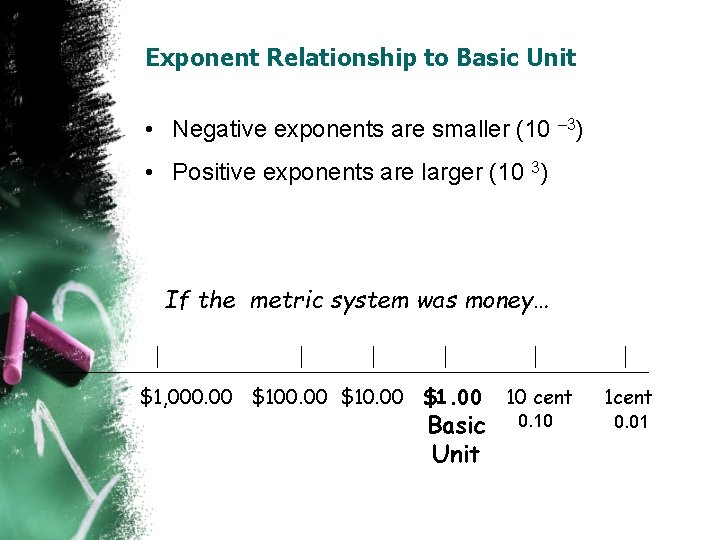
Exponent Relationship to Basic Unit • Negative exponents are smaller (10 – 3) • Positive exponents are larger (10 3) If the metric system was money… | | $1, 000. 00 $10. 00 $1. 00 Basic Unit | | 10 cent 1 cent 0. 10 0. 01
One more point regarding units of measure.
Why is m. L and cc (cm 3) the same? • Cubic centimeter (cc or cm 3) and millimeter (m. L) are used interchangeably in medicine. Ø The unit cc is a length measurement. Ø The unit m. L is a volume measure. • A cube 1 cm long x 1 cm wide by 1 cm high (l x w x h = area) will hold 1 m. L of liquid volume. • We therefore use the units interchangeably. Ø 1 cc or cm 3 = 1 m. L
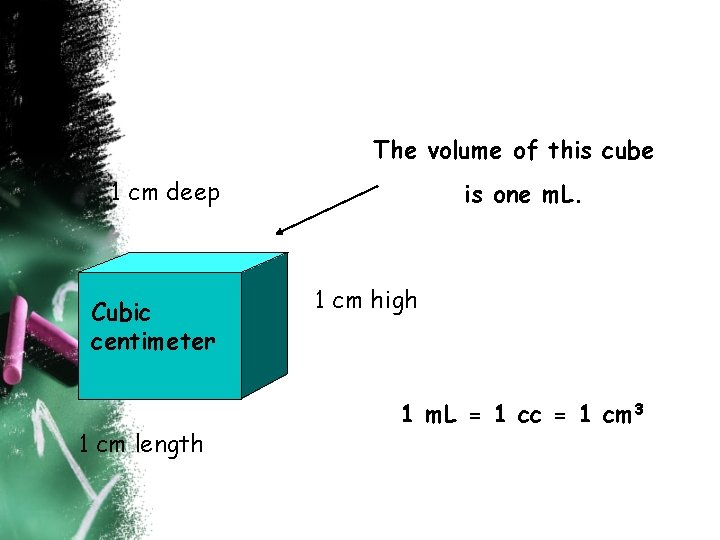
The volume of this cube 1 cm deep Cubic centimeter 1 cm length is one m. L. 1 cm high 1 m. L = 1 cc = 1 cm 3

Additional Conversion Factors Length: 1 meter = 39. 37 inches 1 cm = . 3937 inches 1 km = 0. 62 miles Volume: 1 m. L = 1 cc = 1 L = 1. 0567 qts. 946 m. L = 1 qt. 1 pint = 473 m. L 1 kg = 2. 2 pounds (lbs) 1 lb = 454 grams 1 cm 3
- Slides: 30