Module 6 Part 2 Understanding Advantages of Control






- Slides: 13

Module 6 Part 2 Understanding Advantages of Control Charts for Improvement Science Adapted from: The Institute for Healthcare Improvement (IHI), the Agency for Healthcare Research and Quality (AHRQ), and the Health Resources and Services Administration (HRSA) Quality Toolkits

Objectives • Review how to set, when to revise limits on a control chart • Depict the following data elements on a control chart: –Stratified variable –Rational subgroups –Targets or goals

Criteria for Setting and Locking Control Limits 1. Start calculating control limits after you have 5 points 2. Recalculate the control limits after each point until you reach 20 –Then "lock" these control limits; use them to judge process behavior –If stable, control limits will not change much from point 5 to point 20 –Keywords are "fairly stable" (few, if any, out of control points present 3. Recalculate the control limits again after you have 100 individual results

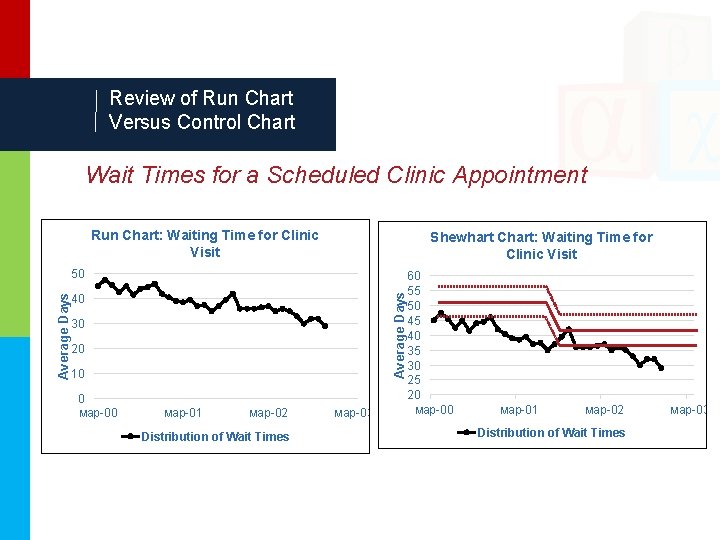
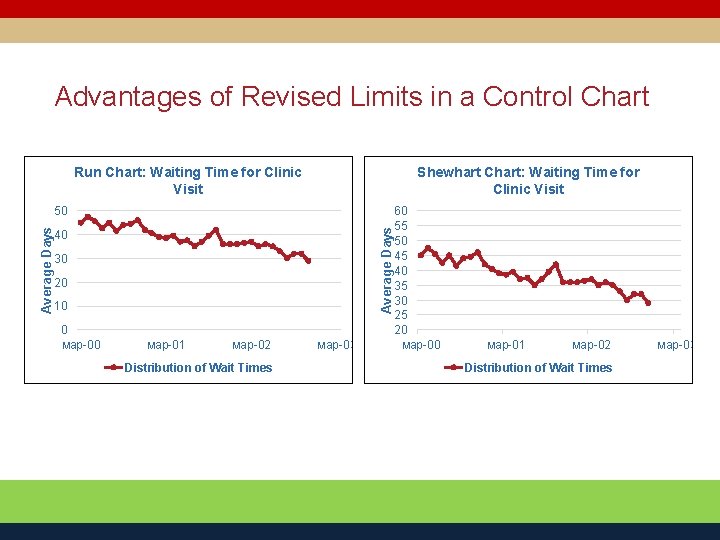
Review of Run Chart Versus Control Chart Wait Times for a Scheduled Clinic Appointment Run Chart: Waiting Time for Clinic Visit Shewhart Chart: Waiting Time for Clinic Visit Average Days 50 40 30 20 10 0 мар-01 мар-02 Distribution of Wait Times мар-03 60 55 50 45 40 35 30 25 20 мар-01 мар-02 Distribution of Wait Times мар-03

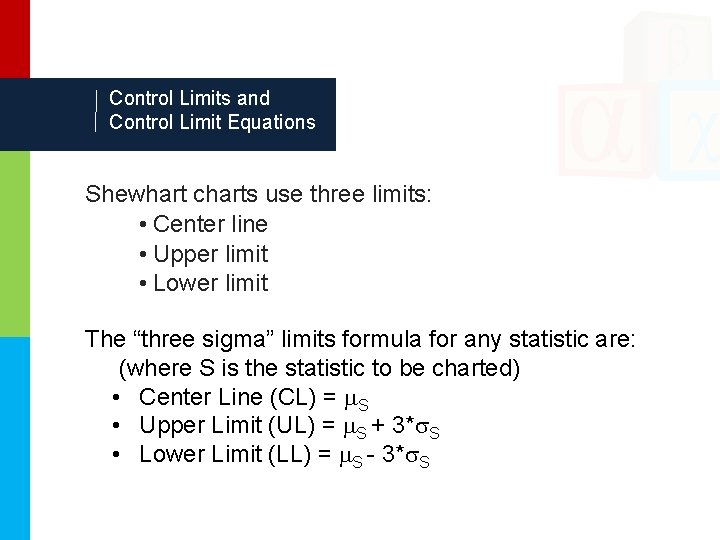
Control Limits and Control Limit Equations Shewhart charts use three limits: • Center line • Upper limit • Lower limit The “three sigma” limits formula for any statistic are: (where S is the statistic to be charted) • Center Line (CL) = S • Upper Limit (UL) = S + 3* S • Lower Limit (LL) = S - 3* S

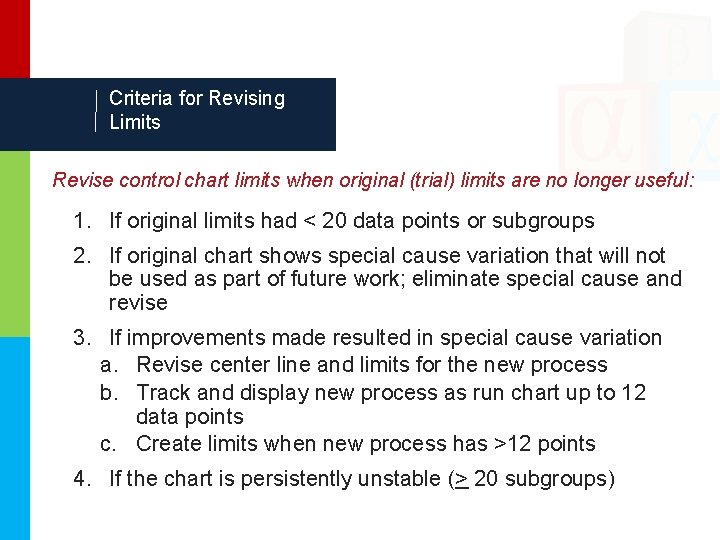
Criteria for Revising Limits Revise control chart limits when original (trial) limits are no longer useful: 1. If original limits had < 20 data points or subgroups 2. If original chart shows special cause variation that will not be used as part of future work; eliminate special cause and revise 3. If improvements made resulted in special cause variation a. Revise center line and limits for the new process b. Track and display new process as run chart up to 12 data points c. Create limits when new process has >12 points 4. If the chart is persistently unstable (> 20 subgroups)

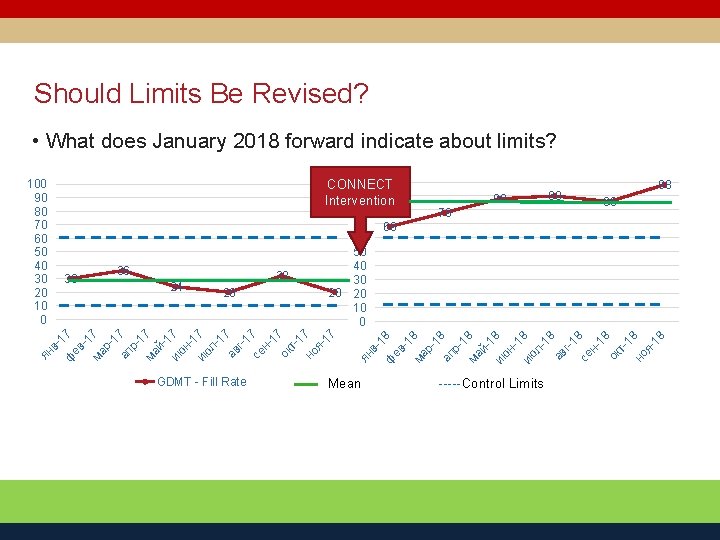
Should Limits Be Revised? • What does January 2018 forward indicate about limits? 100 CONNECT 90 Intervention 36 24 32 20 GDMT - Fill Rate 78 в- Mean 90 98 86 68 ян ян в- 1 ф 7 ев -1 ма 7 р1 ап 7 р1 ма 7 й 1 ию 7 н 1 ию 7 л 17 ав г-1 се 7 н 17 ок т1 но 7 я 17 30 80 70 60 50 40 30 20 20 10 0 88 1 ф 8 ев -1 ма 8 р1 ап 8 р1 ма 8 й 1 ию 8 н 1 ию 8 л 18 ав г-1 8 се н 18 ок т18 но я 18 100 90 80 70 60 50 40 30 20 10 0 -----Control Limits

Advantages of Revised Limits in a Control Chart Run Chart: Waiting Time for Clinic Visit Shewhart Chart: Waiting Time for Clinic Visit Average Days 50 40 30 20 10 0 мар-01 мар-02 Distribution of Wait Times мар-03 60 55 50 45 40 35 30 25 20 мар-01 мар-02 Distribution of Wait Times мар-03

Stratification • THINK – how could I SEE variation best? • Definition: to separate data according to key factors –Male, Female –Smokers, Non-Smokers –Clinic or Hospital A, B or C –Provider A, B or C • Objective: to find patterns associated with causes of process variation

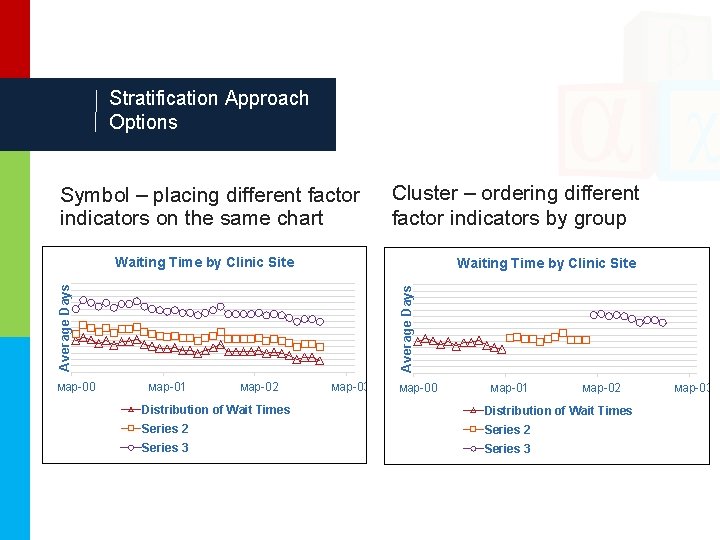
Stratification Approach Options Symbol – placing different factor indicators on the same chart Cluster – ordering different factor indicators by group Waiting Time by Clinic Site мар-00 Average Days Waiting Time by Clinic Site мар-01 мар-02 мар-03 мар-00 мар-01 мар-02 Distribution of Wait Times Series 2 Series 3 мар-03

Rational Subgroups • THINK – Why might the outcome or process vary? • AIM –Include common causes of variation in a subgroup –Observe special causes of variation between subgroups –Hold time constant within a subgroup • Examples of Rational Subgroups –GDMT: provider, hospital/clinic, pharmacy source, payer –ICD Placement: patient preference, provider, duration of HF

Targets and Goals THINK – Is the process moving toward the GOAL Opportunity. Based Composite Score for Adherence to Quality Metrics Evidence-based β-blockers at ≥ 50% target dose ACE-I, ARB, or sacubitril/valsartan use at ≥ 50% target dose Aldosterone antagonist use Anticoagulation use in patients with atrial fibrillation In patients with LVEF ≤ 35%, ICD placement including CRT for patients with sinus rhythm, a LBBB, and a QRS ≥ 150 ms Attendance at 1 or more: multidisciplinary HF disease management program, cardiac rehabilitation program, or HF group educational classes

Summary • Control chart limits should be revised when they no longer serve an interpretive purpose! • Control limits are established to differentiate special cause from common cause variation in a process • Control limits are “locked” once a process is stable • Control limits should be re-set after a process change is implemented and the data show a consistent (stable) shift • Control limits are the only way to determine if a change has been an improvement! • Use of stratification allows better visualization of variability • Use of rational subgroups allows better visualization of cause or source