MODULE 5 Pharmaceutics 1 RA 8203 was signed

























































































- Slides: 101

MODULE 5 Pharmaceutics

1. RA 8203 was signed into law on: A. September 4, 1996 D. September 13, 1986 B. September 13, 1988 E. September 4, 1992 C. October 4, 1996

2. A complete pharmacy internship program based on RA 5921 article III section 18 c shall consist of at least: A. 480 hours B. 960 hours C. 160 hours D. 980 hours E. 940 hours

3. This means a method of secret writing that substitutes other letters or characters for the letter intended, or transpose the letter after arranging them in blocks or squares. A. Code B. Cipher C. Secret Keys D. Substitution E. NOTA CODE – system of words or other systems arbitrarily used to present words SECRET KEYS – characteristic styles or symbols kept from the knowledge of others or disclosed confidentially to only one or a few

4. RA 3720 is also known as: A. B. C. D. E. Pharmacy Law Consumer Act of the Philippines Food, Drugs, Cosmetics and Devices Act Senior Citizens Act Special Law on Counterfeit Drugs

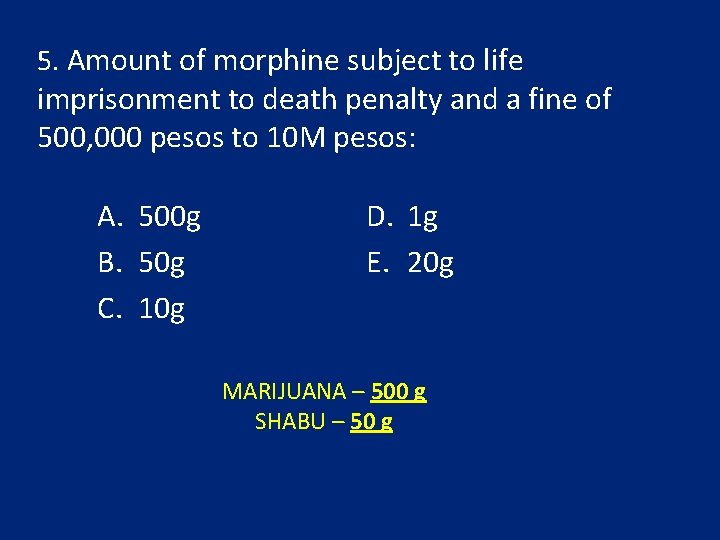
5. Amount of morphine subject to life imprisonment to death penalty and a fine of 500, 000 pesos to 10 M pesos: A. 500 g B. 50 g C. 10 g D. 1 g E. 20 g MARIJUANA – 500 g SHABU – 50 g

6. Ratio of the weight of a substance to the weight of an equal volume of another substance taken as a standard. A. Density B. Specific gravity C. Optical Activity D. Refractive index

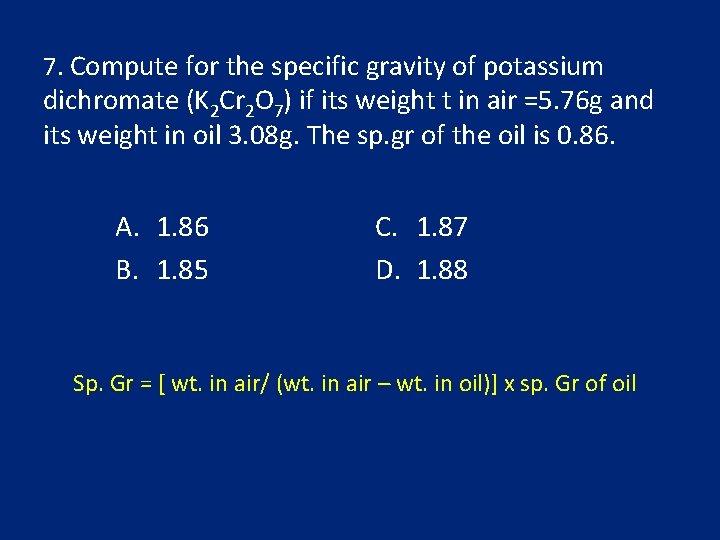
7. Compute for the specific gravity of potassium dichromate (K 2 Cr 2 O 7) if its weight t in air =5. 76 g and its weight in oil 3. 08 g. The sp. gr of the oil is 0. 86. A. 1. 86 B. 1. 85 C. 1. 87 D. 1. 88 Sp. Gr = [ wt. in air/ (wt. in air – wt. in oil)] x sp. Gr of oil

8. Floatation method for the determination of specific gravity is based on whose scientist’s principle? A. Aristotle B. Newton C. Einstein D. Archimedes

9. A type of hydrometer which is used to determine the alcohol strength of any liquid containing only alcohol and water. A. Falle’s hydrometer B. Baume hydrometer C. Thalles’ hydrometer D. Nicholson’s hydrometer Baume hydrometer – used for liquid heavier or lighter than water Nicholson’s hydrometer – type of hydrometer with constant depth of immersion but variable weight. Other examples of such will be Fahrenheit hydrometer, Lovi’s bead

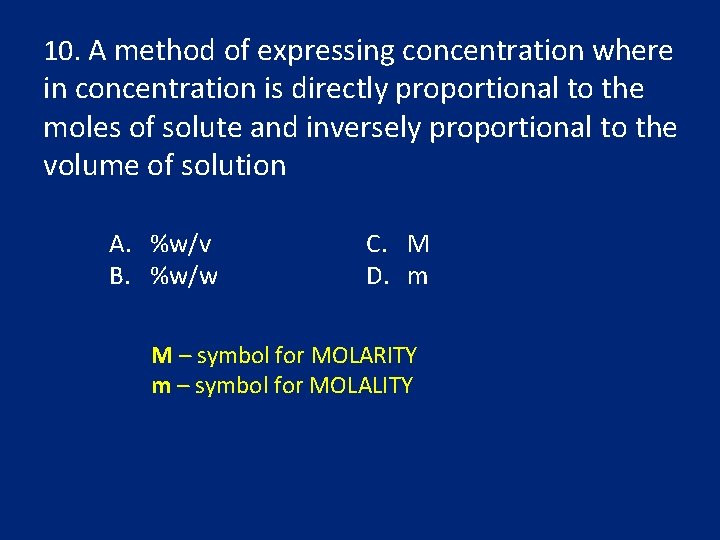
10. A method of expressing concentration where in concentration is directly proportional to the moles of solute and inversely proportional to the volume of solution A. %w/v B. %w/w C. M D. m M – symbol for MOLARITY m – symbol for MOLALITY

11. The p. H value is calculated mathematically as the: A. Log of the hydroxyl ion (OH-) concentration. B. Negative log of the OH- concentration. C. Log of the hydrogen ion (H+) concentration. D. Negative log of the H+ concentration. E. Ratio of H+ /OH- concentration.

12. Which property is classified as colligative? A. Solubility of a solute B. Osmotic pressure C. Hydrogen ion (H+) concentration D. Dissociation of a solute E. Miscibility of the liquids

13. Which equation is used to predict the stability of a drug product at room temperature from experiments at accelerated temperatures? A. Stokes equation B. Young equation C. Arrhenius equation D. Michaelis-Menten equation E. Hixson-Crowel l equation

14. The p. H of a buffer system can be calculated with the: A. Noyes-Whitney equation B. Henderson-Hasselbalch equation C. Michaelis-Menten equation D. Young equation E. Stokes equation

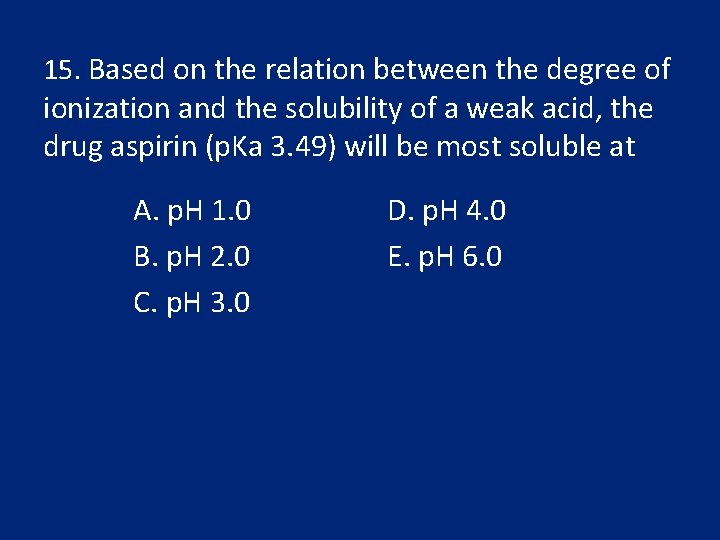
15. Based on the relation between the degree of ionization and the solubility of a weak acid, the drug aspirin (p. Ka 3. 49) will be most soluble at A. p. H 1. 0 B. p. H 2. 0 C. p. H 3. 0 D. p. H 4. 0 E. p. H 6. 0

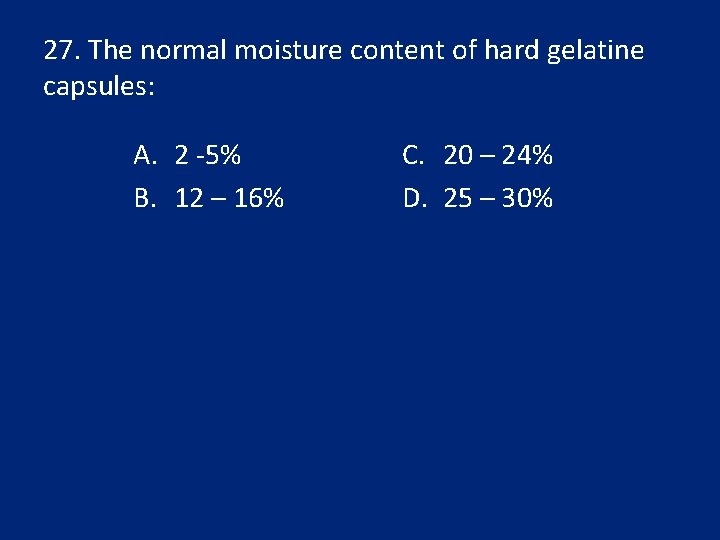
16. Normally, how many % of water is contained in a hard gelatin capsule? A. 8 -10 B. 12 -16 C. 20 -25 D. 2 -5 E. 5 -10

17. The largest size of hard, empty gelatin capsule that can be swallowed is: A. 00 B. 000 C. 1 D. 5 E. 0 The smaller the number the bigger the capsule

18. This chemical agent is used to render the capsule opaque A. Titanium dioxide B. Sorbitol C. Magnesium oxide D. Silica E. Lactose

19. This is a method of preparing tablets in which the powder mixture is compacted in large pieces and subsequently broken down or sized into granules A. Wet granulation B. Dry granulation C. Direct compression In dry granulation, the powder mixture is slugged or compressed into large flat tablets or pellets of about 1 inch in diameter. The slugs are broken up by hand or by the mill and pass through a screen.

20. The following statement/s is/are true for wet granulation method: I. Liquid binder is added to the powder mixture to facilitate the adhesion of the powder particles II. Over-wetting of the powder can result in granules that are too soft for proper tableting and under wetting can result in tablets that are too hard and tend to crumble III. Granules may be dried in thermostatically controlled ovens which constantly record the time, temperature and humidity

21. Amount of shabu subject to life imprisonment to death penalty and a fine of 500, 000 pesos to 10 M pesos: A. 10 g B. 20 g C. 50 g D. 100 g E. 5000 g

22. An ordinary prescription shall be preserved for a period of_______. A. 5 years B. 1 year C. 3 years D. 2 years 5 years – POISON 1 year – DANGEROUS DRUGS

23. When the generic name and brand name are NOT legible, this is a/an______ prescription. A. Violative B. Erroneous C. Impossible D. NOTA Impossible prescription: • Only the generic name is written but is NOT legible • Generic name does NOT correspond with the brand name • Both generic and brand name are NOT legible • Drug product prescribed is NOT registered with the BFAD

24. Supplies or medicines to be given to senior citizen should not exceed A. One day supply B. One week supply C. One month supply D. One year supply

25. Most biological are stored at this temperature: A. 2 – 8°F B. 12 – 8°F C. 2 – 8°C D. 12 – 8°C

26. LAL stands for: A. B. C. D. Limulus Antibiotic Lysate Lyophilized Antibiotic Lysate Limulus Amoebocyte Lysate Lyophilized Amoebocyte Lysate

27. The normal moisture content of hard gelatine capsules: A. 2 -5% B. 12 – 16% C. 20 – 24% D. 25 – 30%

28. When the generic name is not written in a prescription, this is a case of: A. Violative prescription C. Impossible prescription B. Erroneous prescription D. NOTA Violative prescription: • Generic name is NOT written • Generic name is NOT legible and brand name is legible • Brand name is INDICATED and instructions added (e. g. “NO SUBSTITUTION”)

29. “Rule of Thumb” is the principle applied for testing: A. Ampules B. Implantations C. Vials D. Compressed Tablets

30. It refers to the release or movement of the components of the plastic container into the contents: A. Sorption B. Absorption C. Adsorption D. Leaching Sorption and Absorption – refers to the binding of drug molecules to the polymer material of the plastic container

31. It states that vapor pressure of component is equal to its mole fraction times vapor pressure in the pure state at that same temperature. A. Raoult’s law B. Henry’s law C. Charles’ law D. Fick’s law

32. Which of the following is not a colligative property? A. B. C. D. Boiling point elevation Freezing point depression Vapor pressure elevation Osmotic pressure - vapor pressure LOWERING

33. Dissociation constant for substances that dissociates into 5 ions A. 4. 2 B. 1. 8 C. 3. 4 D. 2. 6 B – 2 ions C – 4 ions D – 3 ions

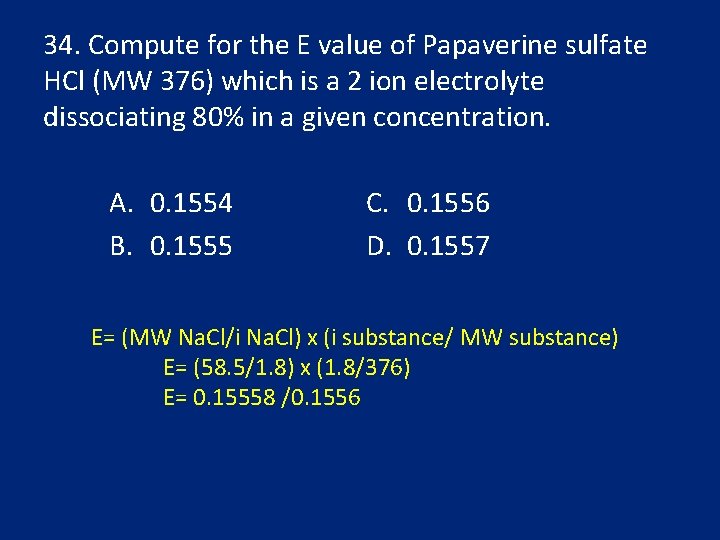
34. Compute for the E value of Papaverine sulfate HCl (MW 376) which is a 2 ion electrolyte dissociating 80% in a given concentration. A. 0. 1554 B. 0. 1555 C. 0. 1556 D. 0. 1557 E= (MW Na. Cl/i Na. Cl) x (i substance/ MW substance) E= (58. 5/1. 8) x (1. 8/376) E= 0. 15558 /0. 1556

35. A simplication of the White-Vincent method where the weight of the drug is fixed in 0. 3 g for one fluidounce of a 1% solution. A. B. C. D. Modified White-Vincent method Scowle’s method Sprowl’s method Cryoscopic method

36. Dosage form made by compression or molding A. Tablets B. Capsule C. Syrup D. Suspension

37. A characteristic of material for compression which describes the way the material flow thru the hopper A. Fluidity B. Compressibility C. Dryness D. Particle size

38. Spray drug lactose when used in direct compression can hold how many % of active ingredient? A. 18% B. 15% C. 20% D. 30% It can hold 20 -25% of the active ingredient

39. Which of the following is not a glidant? A. CAB-O-SIL B. DL-Leucine C. Syloid D. Talc - It is solely used as an anti-adherent. (A), (C), & (D) are used as both glidant and anti-adherent. Glidants - improve flow property. Anti-adherent - prevents sticking & picking.

40. Clays are limitedly used for what type of tablets due to its tendency to cause unwanted appearance? A. White tablets B. Colored tablets C. Both D. NOTA

41. Which solution is used as an astringent? A. Strong iodine solution USP B. Aluminum acetate topical solution USP C. Acetic acid NF D. Aromatic ammonia spirit USP E. Benzalkonium chloride solution NF Aluminum acetate - used as antiperspirants and wet dressings for contact dermatitis Strong iodine and Benzalkonium chloride – topical antibacterial solutions Acetic acid - acidifier Aromatic ammonia spirit – respiratory stimulant

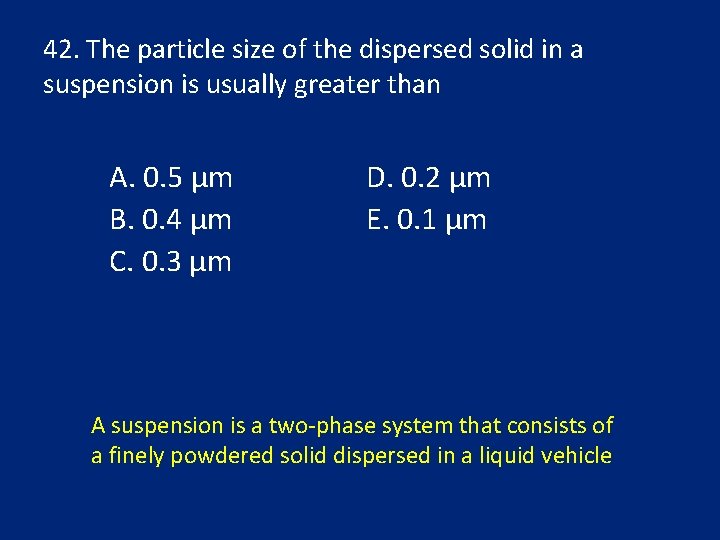
42. The particle size of the dispersed solid in a suspension is usually greater than A. 0. 5 μm B. 0. 4 μm C. 0. 3 μm D. 0. 2 μm E. 0. 1 μm A suspension is a two-phase system that consists of a finely powdered solid dispersed in a liquid vehicle

43. In the extemporaneous preparation of a suspension, levigation is used to: A. reduce the zeta potential B. avoid bacterial growth C. reduce particle size D. enhance viscosity E. reduce viscosity Levigation is the process of blending and grinding a substance to separate the particles, reduce their size, and form a paste.

44. Which compound is a natural emulsifying agent? A. acacia B. lactose C. polysorbate 20 D. polysorbate 80 E. sorbitan monopalmitate

45. Rectal suppositories intended for adult use usually weigh approximately A. 1 g B. 2 g C. 3 g D. 4 g E. 5 g

46. In the fusion method of making cocoa butter suppositories, which substance is most likely to be used to lubricate the mold? A. Mineral oil B. Propylene glycol C. Cetyl alcohol D. Stearic acid E. Magnesium silicate

47. A very fine powdered chemical is defined as one that A. completely passes through a #80 sieve. B. completely passes through a #120 sieve. C. completely passes through a #20 sieve. D. passes through a #60 sieve and not more than 40% through a #100 sieve. E. passes through a #40 sieve and not more than 60% through a #60 sieve.

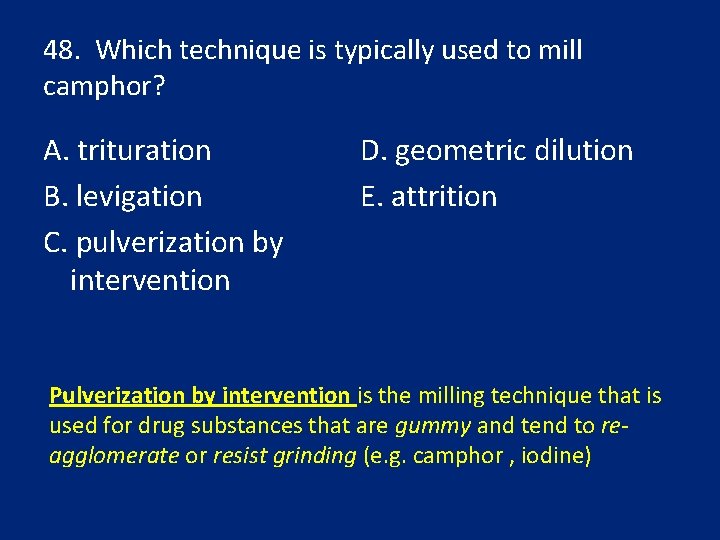
48. Which technique is typically used to mill camphor? A. trituration B. levigation C. pulverization by intervention D. geometric dilution E. attrition Pulverization by intervention is the milling technique that is used for drug substances that are gummy and tend to reagglomerate or resist grinding (e. g. camphor , iodine)

49. The dispensing pharmacist usually blends potent powders with a large amount of diluent by A. spatulation B. sifting C. trituration D. geometric dilution E. levigation

50. Which type of paper best protects a divided hygroscopic powder? A. waxed paper B. glassine C. white bond D. blue bond E. vegetable parchment Hygroscopic and volatile drugs are best protected by waxed paper , which is waterproof.

51. This process is a form of pelletization, which refers to the formation of spherical particles from wet granulation A. Spheronization B. Slugging C. Spray chilling D. Moist heating E. Dry heating • Slugging - a process in which the powder mixture is compressed into large flat tablet or pellet.

52. Which excipient/s is/are used in the preparation of sugar-free chewable tablets? I. Lactose II. Dextrose A. B. C. D. E. I only III only I and II II and III I, III III. Xylitol

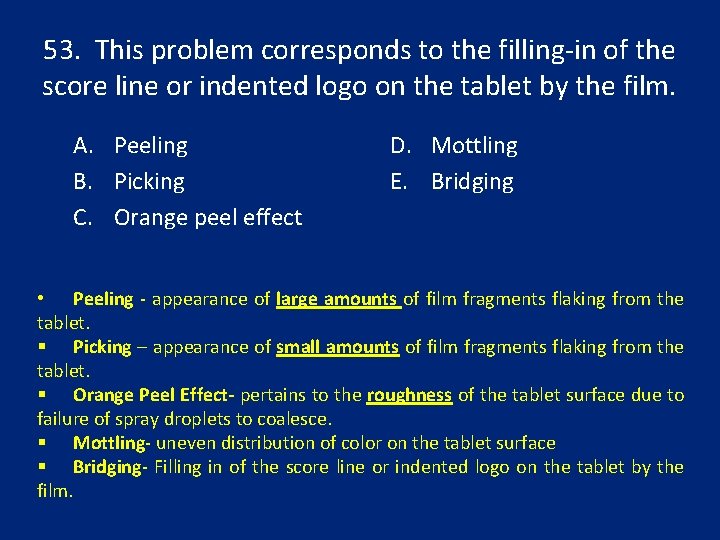
53. This problem corresponds to the filling-in of the score line or indented logo on the tablet by the film. A. Peeling B. Picking C. Orange peel effect D. Mottling E. Bridging • Peeling - appearance of large amounts of film fragments flaking from the tablet. § Picking – appearance of small amounts of film fragments flaking from the tablet. § Orange Peel Effect- pertains to the roughness of the tablet surface due to failure of spray droplets to coalesce. § Mottling- uneven distribution of color on the tablet surface § Bridging- Filling in of the score line or indented logo on the tablet by the film.

54. This type of dosage form allows a reduction in dosing frequency to that presented by a conventional dosage form A. Extended release B. Delayed release C. Repeat Action D. Modified Release E. Targeted Release Delayed release- designed to release the drug from the dosage form at a time other than promptly after administration. Repeat Action- Usually contains 2 doses of medication one for immediate and the second for delayed release. Targeted release - describes drug release directed towards isolating or concentrating a drug in a body region, tissue or site for absorption or for drug action.

55. Yellow ointment is an example of A. Hydrocarbon base B. Oleaginous base C. Absorption base D. Water removable base E. A & B Hydrocarbon base is Oleaginous base

56. The following ointment base/s is/are classified as hydrocarbon base/s I. Petrolatum II. White ointment III. Polyethylene Glycol Ointment A. B. C. D. E. I only I & III III only I, III

57. Polyethylene glycol Ointment, NF is: A. Hydrocarbon base B. Water Soluble base C. Absorption base D. Water Removable base E. Oleaginous base

58. These ointment base resembles cream in appearance A. Hydrocarbon base B. Water Soluble base C. Absorption base D. Water Removable base E. Oleaginous base Water removable bases are oil-in-water emulsion resembling creams in appearance. They are easily washed from skin and often called “water washable” base.

59. These are semi-solid adhesive masses spread upon a backing material paper, fabric, moleskin or plastic A. Cream B. Plaster C. Paste D. Ointment E. Lotion

60. The factor/s which play/s a part in percutaneous absorption is/are: I. Molecular Weight II. Partitioning coefficient III. Solubility A. B. C. D. E. I only I & II II & III I, III

61. In sugar coating of tablets, the greatest increase in the size of the tablet occurs at what stage of the process? A. Sealing B. Smoothing C. Color coating D. Sub coating E. Polishing

62. Uneven distribution of color on the surface of tablets is: A. Peeling B. Mottling C. Capping D. Lamination E. Picking

63. Strength of a product is expressed in terms of: A. Potency B. Activity C. Toxicity D. Therapeutic dose E. Lethal dose

64. An example of a tetragonal crystal system is: A. Urea B. Iodoform C. Iodine D. Sodium chloride Iodoform – hexagonal Iodine – rhombic Sodium chloride - cubic

65. These are properties which are dependent on the arrangement and to a lesser extent on the number and kind of atoms within a molecule: A. Intensive B. Colligative C. Constitutive D. Extensive – DEPENDENT on the quantity of matter in a system Intensive – INDEPENDENT of the quantity of substance in the system (e. g. temperature, density, pressure, surface tension, viscosity) Colligative - e. g. Osmotic pressure elevation, vapor pressure lowering, Freezing point depression, and boiling point elevation

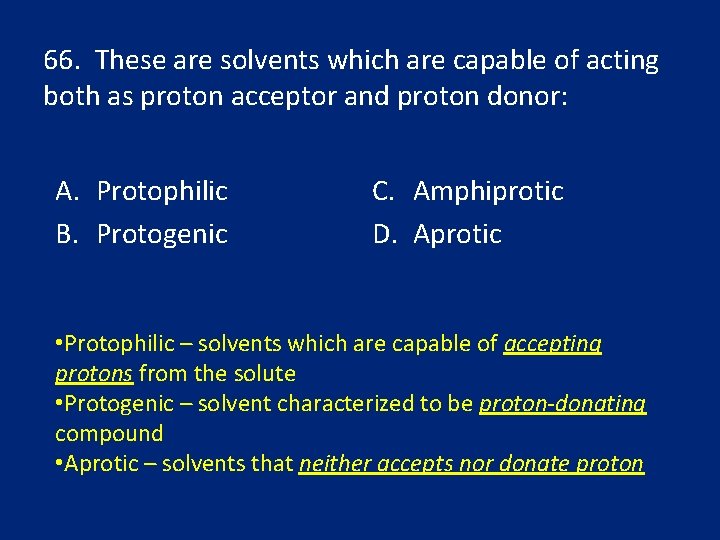
66. These are solvents which are capable of acting both as proton acceptor and proton donor: A. Protophilic B. Protogenic C. Amphiprotic D. Aprotic • Protophilic – solvents which are capable of accepting protons from the solute • Protogenic – solvent characterized to be proton-donating compound • Aprotic – solvents that neither accepts nor donate proton

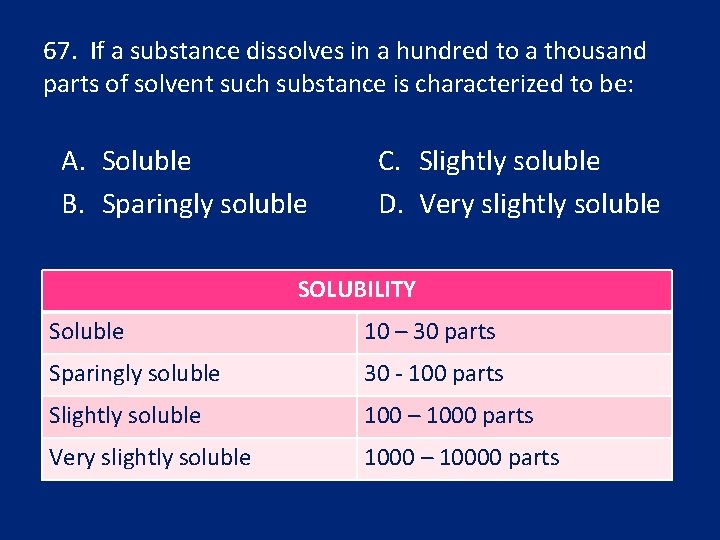
67. If a substance dissolves in a hundred to a thousand parts of solvent such substance is characterized to be: A. Soluble B. Sparingly soluble C. Slightly soluble D. Very slightly soluble SOLUBILITY Soluble 10 – 30 parts Sparingly soluble 30 - 100 parts Slightly soluble 100 – 1000 parts Very slightly soluble 1000 – 10000 parts

68. This is also known as the “shear thinning systems” A. Pseudoplastic B. Dilatant C. Thixotrophic D. Rheopectic Dilatant – “shear thickening system” Thixotrophic – gels and magmas when standing form semisolids and on shaking become fluid

69. A phenomenon where in a gel takes up liquid without an observable increase in volume: A. Sweating B. Syneresis C. Swelling D. Imbibition Syneresis – phenomenon in which the liquid portion of a gel is pressed out Swelling – process which involves the taking up of liquid by a gel with an observable increase in its volume

70. This type of solution has a solute concentration equivalent to its limit of solubility A. Unsaturated B. Saturated C. Supersaturated D. NOTA

71. Breaking down of materials by rubbing action between 2 surfaces. A. Attrition B. Rolling C. Impact D. Scratching Rolling - uses heavy rolling to crush materials. Impact - employs hammer/bars at high speed to break material.

72. All are type of plastic containers, EXCEPT; A. PVC B. PET C. PP D. PD PVC - polyvinyl chloride PET – polyethylene PP - polypropylene

73. At what percent is is benzalkonium chloride used? A. 0. 2% B. 0. 1% C. 0. 001% D. 0. 002%

74. A cool place would be A. <8 C B. 2 -8 C C. 8 -15 C D. -20 - -15 C

75. Composition of the council of pharmaceutical education, EXCEPT; A. B. C. D. Secretary of Education Dean of UP College of Pharmacy BFAD DOST Secretary

76. Qualification for Board examination, except; A. B. C. D. E. Citizen of the Philippines Moral character Completed internship BS Pharmacy graduate NOTA

77. Revocation of LTO may be due to the following except; A. B. C. D. E. Selling of drug with “not for sale” mark Failure to properly record dangerous drugs Absence of pharmacist AOTA NOTA

78. RA 7432 A. Senior Citizen’s Act B. Pharmacy Law C. Generic’s Act D. Dangerous Drugs Act

79. RA 6425 A. Senior Citizen’s Act B. Pharmacy Law C. Generic’s Act D. Dangerous Drugs Act

80. RA 6675 A. Senior Citizen’s Act B. Pharmacy Law C. Generic’s Act D. Dangerous Drugs Act

81. A certain drug is considered counterfeit if it contains less than how many percent of the active ingredient it purports to posses? A. 80 B. 75 C. 60 D. 85 E. 90 Counterfeit drug/medicine refers to a drug which contains no amount of, or different active ingredients or less than eighty percent of the active ingredient it purports to possess (RA 8203, Section 3)

82. For how many years shall the Chairman and members of the Board of Pharmacy hold office after appointment? A. 3 B. 4 C. 5 D. 6 E. 2

83. Any person who shall be employed as detailman by any pharmaceutical establishment shall be required to register with the: A. B. C. D. E. PRC BFAD Board of Pharmacy DOH Council of Pharmaceutical Education

84. Every prescription for external use filled in the drugstore shall bear what label? A. Red label B. White label C. Red label showing in blank ink the components and the word “External Use Only” D. White label showing red ink the Word “External Use Only” E. NOTA

85. The pharmacist examination shall consist of theoretical examination on the subjects in: A. Chemistry B. Biological Science C. Pharmacy D. A and C Only E. AOTA

86. The book kept for the purpose of recording the sale of poisons shall be open at all timed for inspection and shall be preserved for a period of at least _____ years after the last entry. A. 1 B. 2 C. 3 D. 5 E. 7

87. The following substances are included in the list of violent poisons stated in RA 5921: A. Atropine B. Nitrobenzene C. Strychnine D. B and C only E. AOTA Also include in the list are arsenical preparations, phosphorus, corrosive sublimate, hydrocyanic acid or prussic acid and such other poisonous substances which may be classified as violent by BFAD (RA 5921, Article IV, Section 34)

88. This means a system of words or other systems arbitrary used to present words. A. Code B. Cipher C. Secret Keys D. Substitution E. NOTA

89. The term Secretary, in RA 3720, means: A. B. C. D. Secretary of DOH Secretary of Dept of Commerce and Industry Secretary of Education Secretary of Department of Agricultural Resources E. AOTA

90. Under RA 3720, if a food bears or contains any poisonous or deleterious substances which may render it injurious to health, it is deemed to be: A. Poison B. Adulterated C. Misbranded D. Counterfeit E. Hazardous

91. If the seized drug was found to be counterfeit, the business establishment must be directed for preventive closure for a period of: A. 15 days B. 10 days C. 30 days D. 20 days E. 60 days

92. If the counterfeit drug is the proximate cause of death of the victim who unknowingly purchased and took the counterfeit drug, the penalty to be imposed shall be: A. B. C. D. E. Life imprisonment Fine of 500, 000 to 5 M Pesos Fine of 100, 000 to 10 M Pesos A & B Only A & C only

93. The council of Pharmaceutical Education was created for the implementation of RA 5921. This council is composed of: A. B. C. D. E. Secretary of Education BFAD administrator Chairman of the Board of Pharmacy A&C AOTA Other members are Undersecretary of Health Service, Dean of UP College of Pharmacy, Deans of the Colleges of Pharmacy representing duly, accredited private schools of pharmacy and a representative of a bona fide national pharmaceutical organization of the Philippines.

94. If found guilty of neglect of duty, incompetence, malpractice or unprofessional or dishonorable conduct, after having been given the opportunity to defend himself in an administrative investigation, the Chairman or members of the Board may be removed by: A. President of the Philippines B. Chairman of the Council of Pharmaceutical Education C. BFAD administrator D. PRC Comissioner E. Civil Service Commissioner

95. Any person who shall be employed as detailman by any pharmaceutical establishment shall be required to register with the A. B. C. D. E. PRC BFAD Board of Pharmacy DOH Council of Pharmaceutical Education

96. Every prescription for external use filled in the drugstore shall bear what label A. Red label B. White label C. Red label showing in black ink the component and the words “For external use only” D. White label showing in red ink the word “For external use only” E. NOTA

97. The book kept for the purpose of recording the sale of poisons shall be open at all times for the inspection and shall be preserved for a period of at least ____ years after the last entry A. 1 B. 2 C. 3 D. 5 E. 7

98. RA 3720 was signed into law on: A. June 22, 1963 B. June 22, 1969 C. June 23, 1969 D. July 22, 1969 E. July 22, 1963

99. Prescription of Dangerous Drugs are written in: A. Duplicate B. Triplicate C. One copy D. Four copies E. NOTA one original, 2 duplicates ( Original- pharmacist, 1 copy- patient, 1 copy- practitioner)

100. Head of the PDEA A. Secretary of Health D. PNP Chief E. BFAD Director B. Director General C. NBI Chief