Module 4 Biochemistry Section 2 Amino Acids and
Module 4 Biochemistry Section 2: Amino Acids and Proteins 1

Key Concepts • • Functions of Proteins Classification of Amino Acids Properties and Interactions of Amino Acids Four Levels of Protein Structure – Description and Distinguishing Features • Factors Influencing Protein Folding and Stability • Examples of Proteins and Their Structural Features 2

Proteins • Polymers of amino acids • Vary in length from a few to several thousand amino acids. • Molecular weight ranges from a few hundred to half -million • Proteins exist independently in solution, as complexes with small molecules (ligands) or other proteins, and as integral components of cell membranes. 3
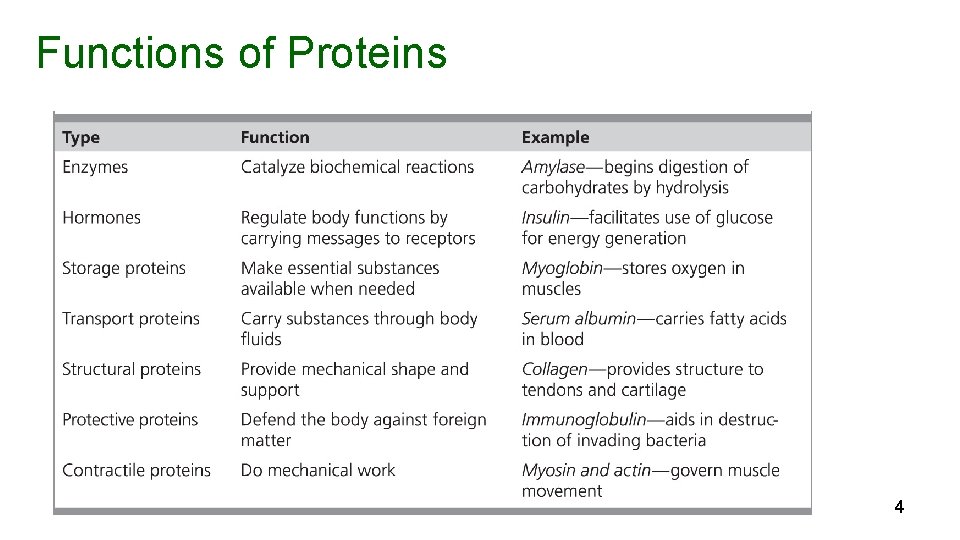
Functions of Proteins 4

Amino Acids Nearly all proteins in plants and animals are composed of the 20 “common” -amino acids. 10 (nonessential) can be synthesized in the body) and 10 (essential) must be obtained from the diet. 5
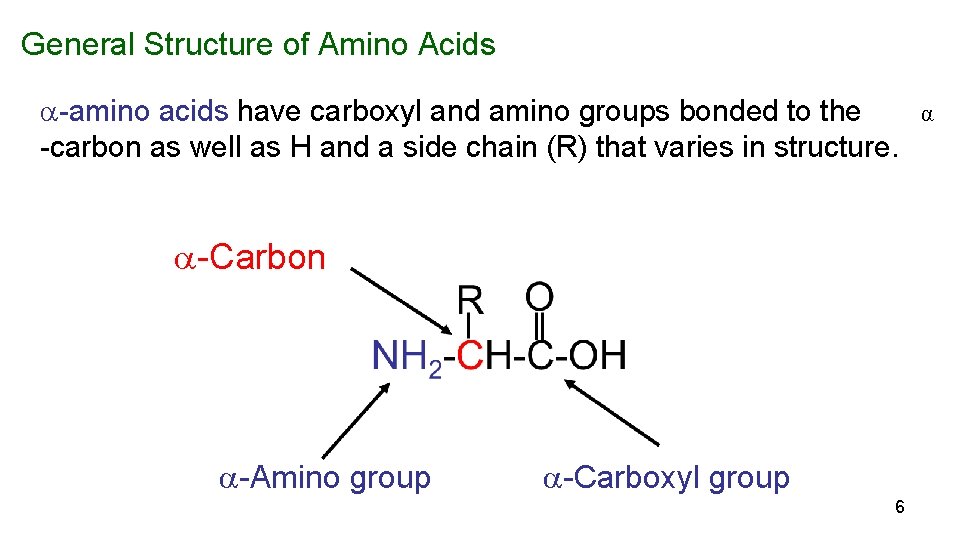
General Structure of Amino Acids -amino acids have carboxyl and amino groups bonded to the -carbon as well as H and a side chain (R) that varies in structure. -Carbon -Amino group -Carboxyl group 6 α
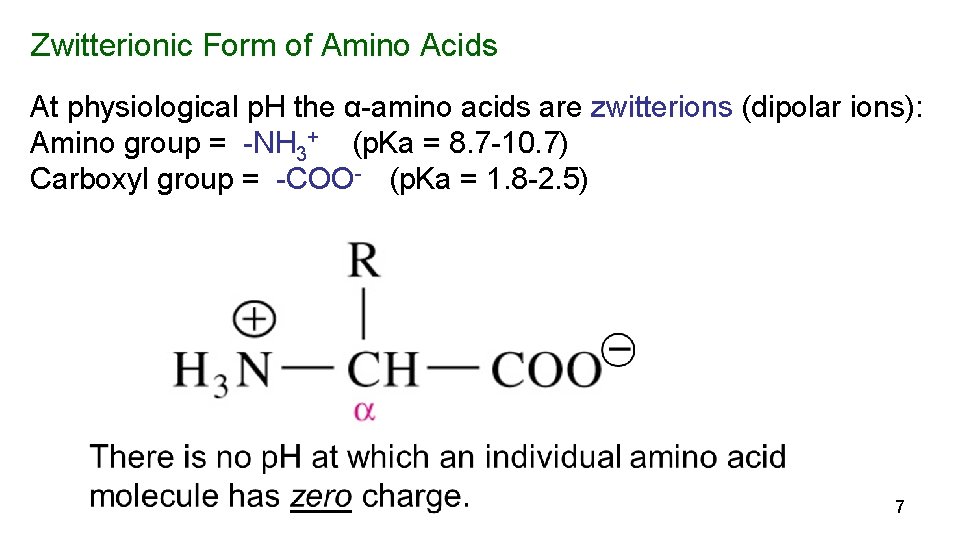
Zwitterionic Form of Amino Acids At physiological p. H the α-amino acids are zwitterions (dipolar ions): Amino group = -NH 3+ (p. Ka = 8. 7 -10. 7) Carboxyl group = -COO- (p. Ka = 1. 8 -2. 5) 7
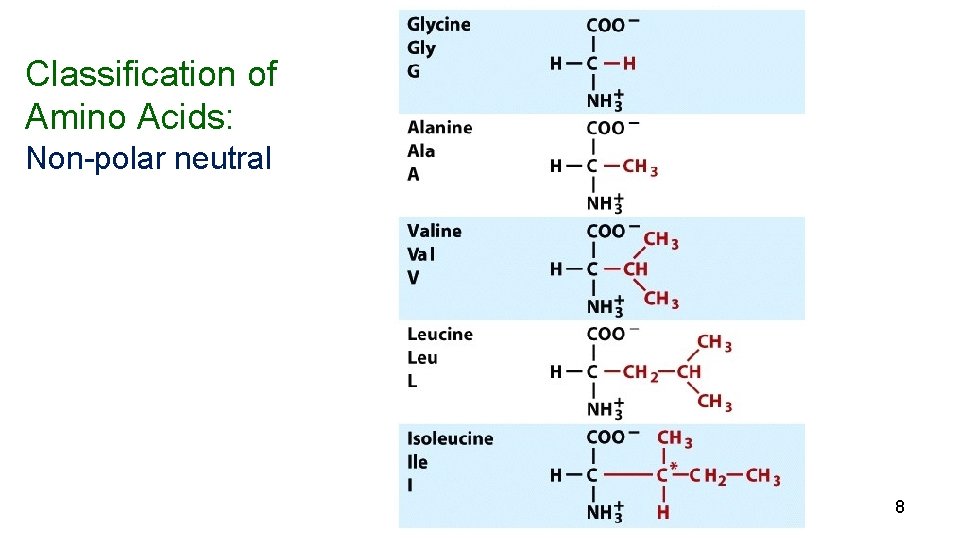
Classification of Amino Acids: Non-polar neutral 8
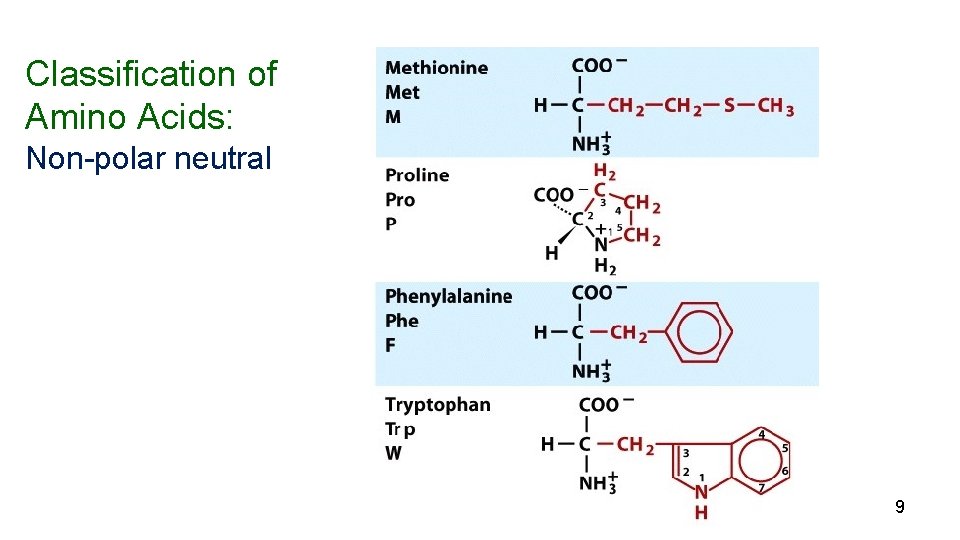
Classification of Amino Acids: Non-polar neutral 9
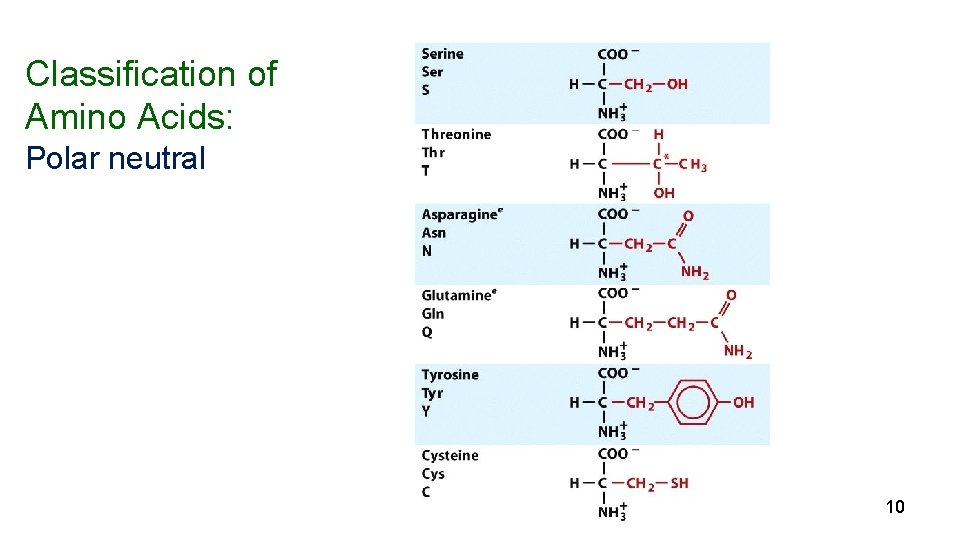
Classification of Amino Acids: Polar neutral 10
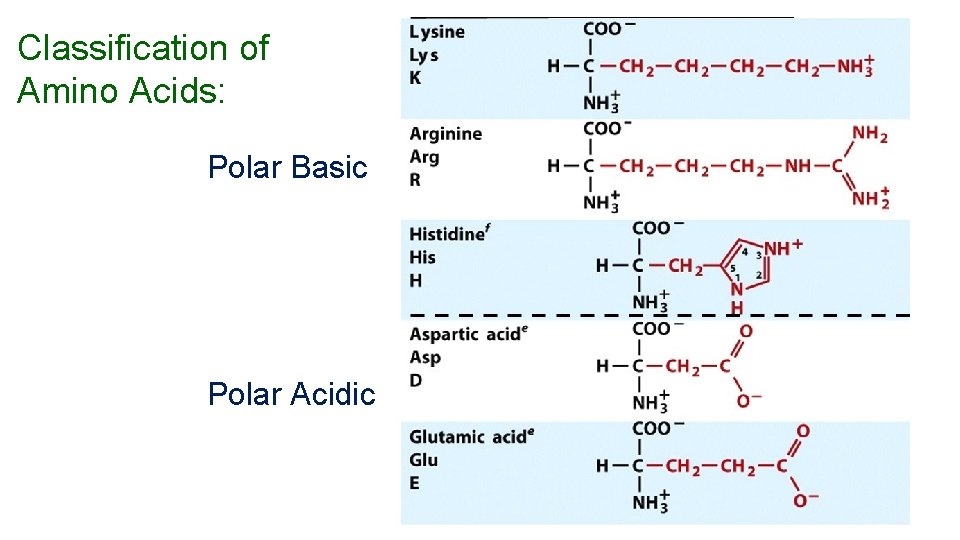
Classification of Amino Acids: Polar Basic Polar Acidic 11
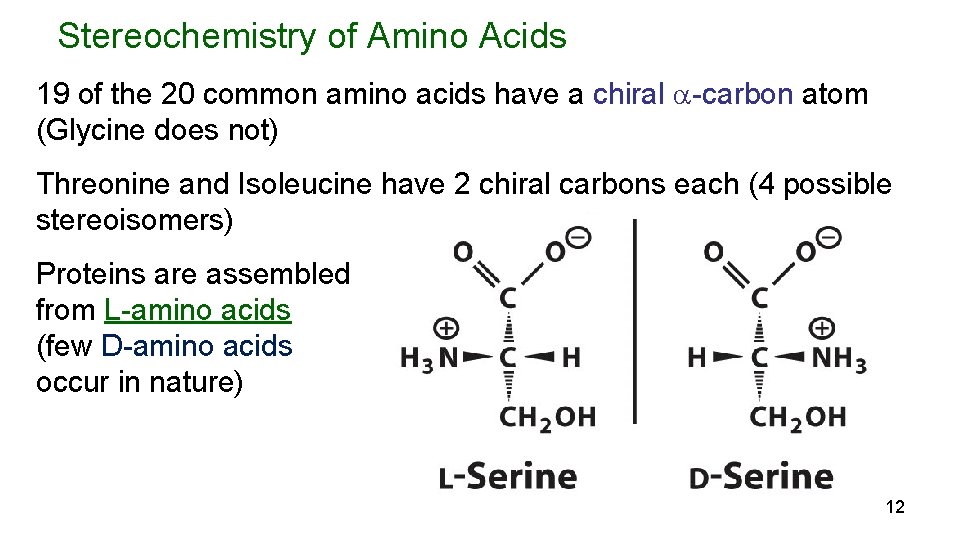
Stereochemistry of Amino Acids 19 of the 20 common amino acids have a chiral -carbon atom (Glycine does not) Threonine and Isoleucine have 2 chiral carbons each (4 possible stereoisomers) Proteins are assembled from L-amino acids (few D-amino acids occur in nature) 12

Peptide Bonds Link Amino Acids in Proteins Peptide bond – the linkage between amino acids is a secondary amide bond. Formed by condensation of the -carboxyl of one amino acid with the -amino group of another (with loss of H 2 O). 13
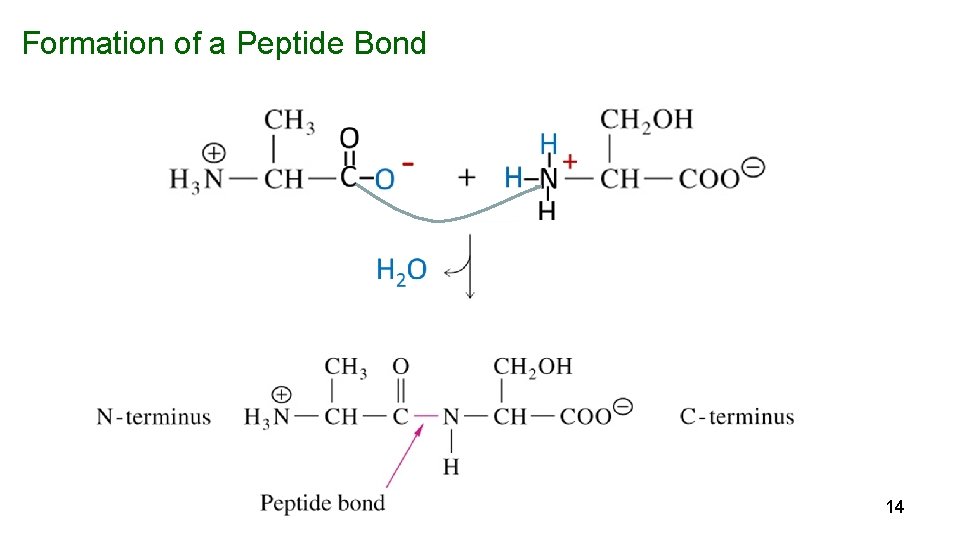
Formation of a Peptide Bond 14
Polypeptide Nomenclature • Individual amino acids within a polypeptide chain are called “residues” • Formation of peptide bonds eliminates the ionizable α-carboxyl and α-amino groups of the constituent amino acids except for the N(amino) and C- (carboxy) terminal residues. • Shorthand sequences are written from the N-terminus to the Cterminus, i. e. Gly-Arg-Phe-Ala-Lys (or GRFAK) • Di-, tri-, tetrapeptides (<5 residues); oligopeptides (5 -20 residues); polypeptides and proteins (>20 residues) 15
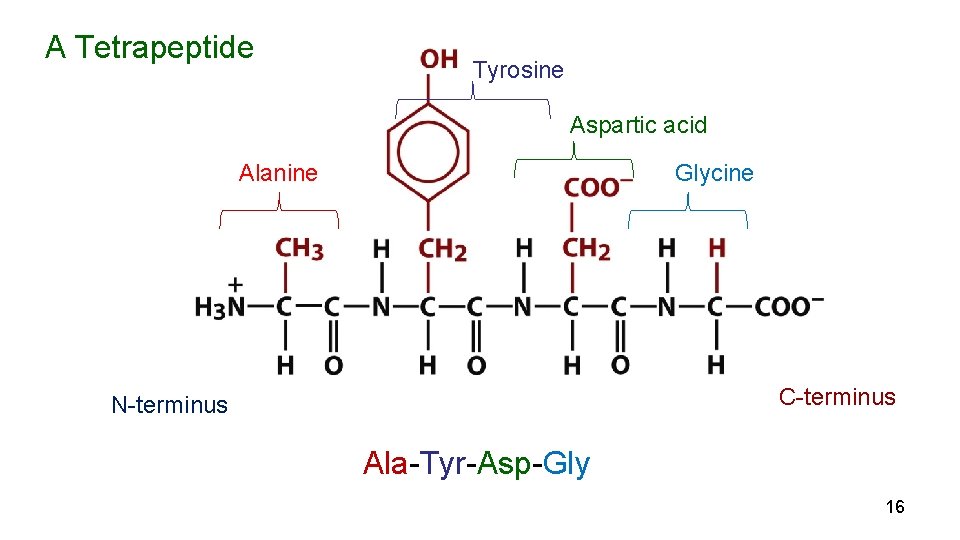
A Tetrapeptide Tyrosine Aspartic acid Glycine Alanine C-terminus N-terminus Ala-Tyr-Asp-Gly 16
Interactions Between Amino Acids Interactions of amino acid side chains in proteins are important for: • Maintaining protein structure • Binding small molecules (ligands) • Facilitating interactions with other proteins • Forming catalytic “active” sites in enzymes • Regulating enzyme activity 17
Interactions Between Amino Acids 1) Van der Waals Interactions: Weak non-covalent interaction between any two atoms in close proximity. Temporary dipoles in one molecule induce the opposite dipole in the approaching molecule. 2) The Hydrophobic Effect: Nonpolar molecules and nonpolar regions of molecules tend to associate with each other to exclude polar water molecules. This stabilizing interaction is called the ‘hydrophobic effect’. 18
Interactions Between Amino Acids 3) Hydrogen Bonding: A type of dipole-dipole interaction. Formed between the H in X-H, where X is an electronegative atom (O, N, S), and Y, which is a second electronegative atom (O, N, S). Most common H-bonds in biological molecules occur where X and Y are N and/or O. 4) Charge-Charge Interactions: Oppositely charged amino acids participate in electrostatic attractions. Interaction between similarly charged amino acids is strongly repulsive. 19
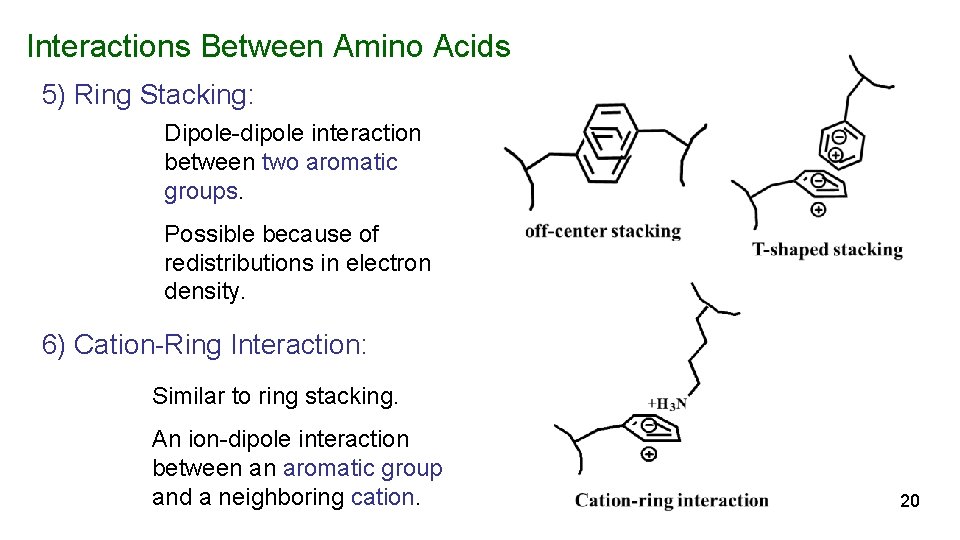
Interactions Between Amino Acids 5) Ring Stacking: Dipole-dipole interaction between two aromatic groups. Possible because of redistributions in electron density. 6) Cation-Ring Interaction: Similar to ring stacking. An ion-dipole interaction between an aromatic group and a neighboring cation. 20
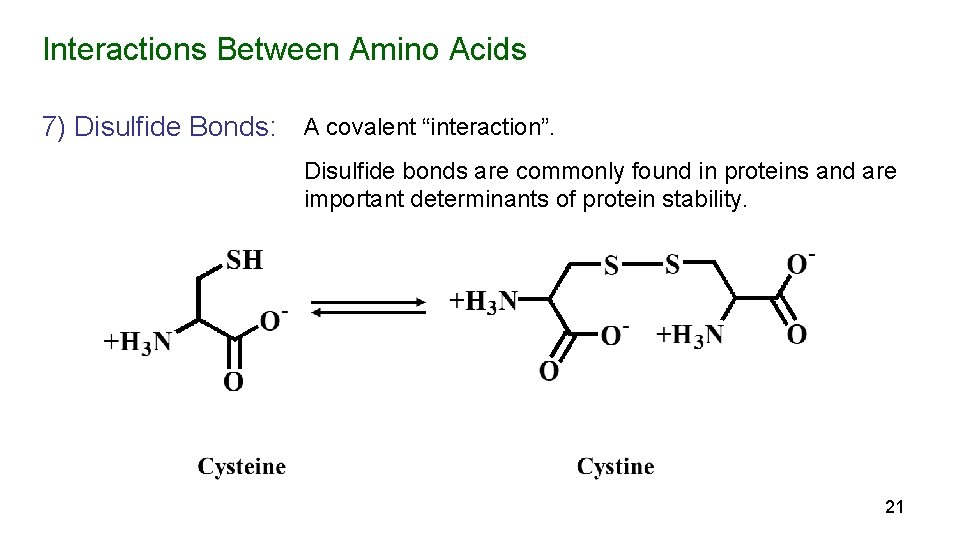
Interactions Between Amino Acids 7) Disulfide Bonds: A covalent “interaction”. Disulfide bonds are commonly found in proteins and are important determinants of protein stability. 21
Four Levels of Protein Structure • Primary structure - amino acid sequence • Secondary structure - regions of local recognizable conformations of the polypeptide chain, such as helices and b-strands (form b-sheets). • Tertiary structure - combinations of secondary structural elements that together determine the 3 -D shape of the fully folded polypeptide chain • Quaternary structure - arrangement of two or more polypeptide chains into a multichain (multisubunit) structure 22
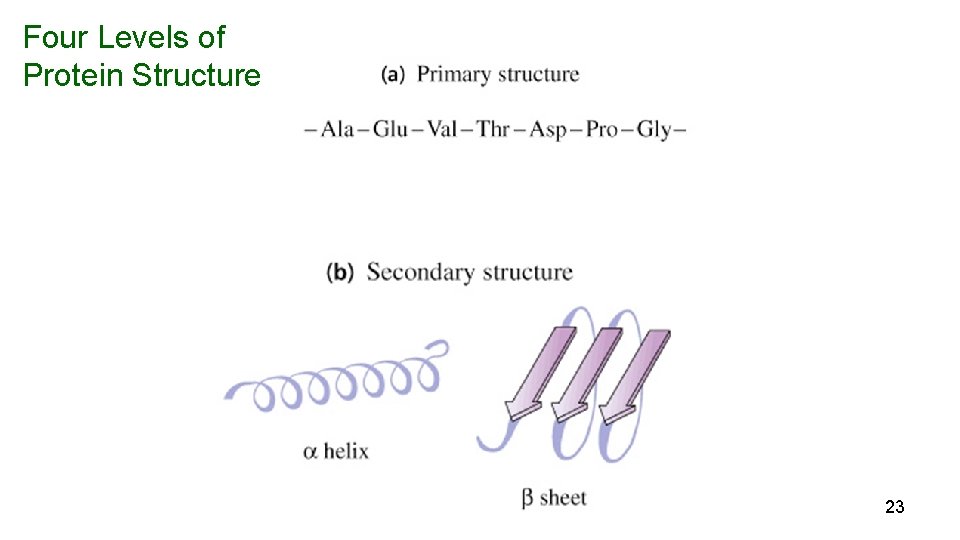
Four Levels of Protein Structure 23
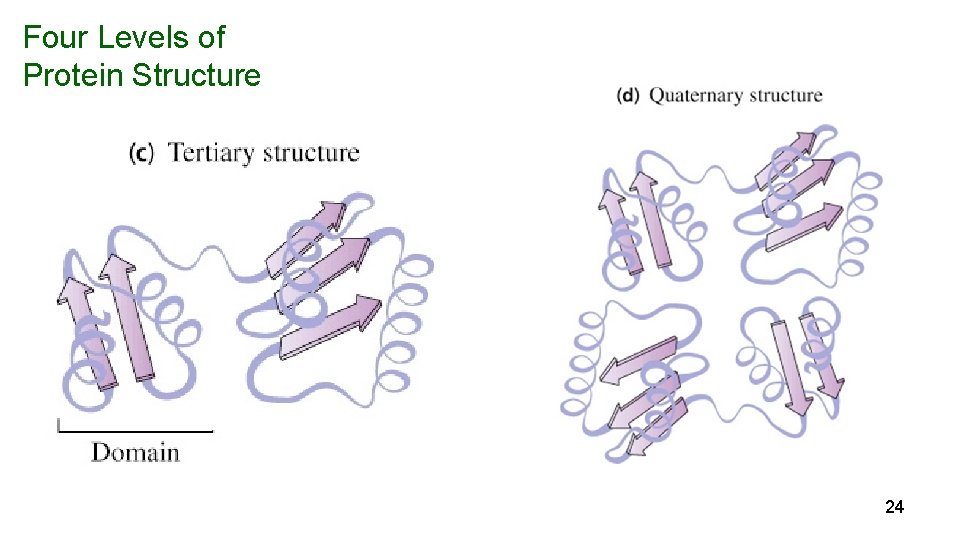
Four Levels of Protein Structure 24
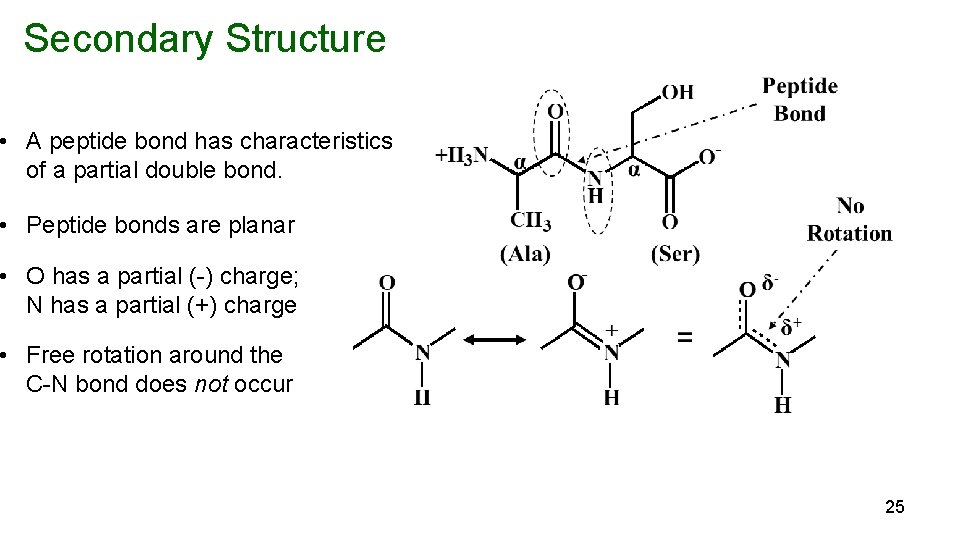
Secondary Structure • A peptide bond has characteristics of a partial double bond. • Peptide bonds are planar • O has a partial (-) charge; N has a partial (+) charge • Free rotation around the C-N bond does not occur 25
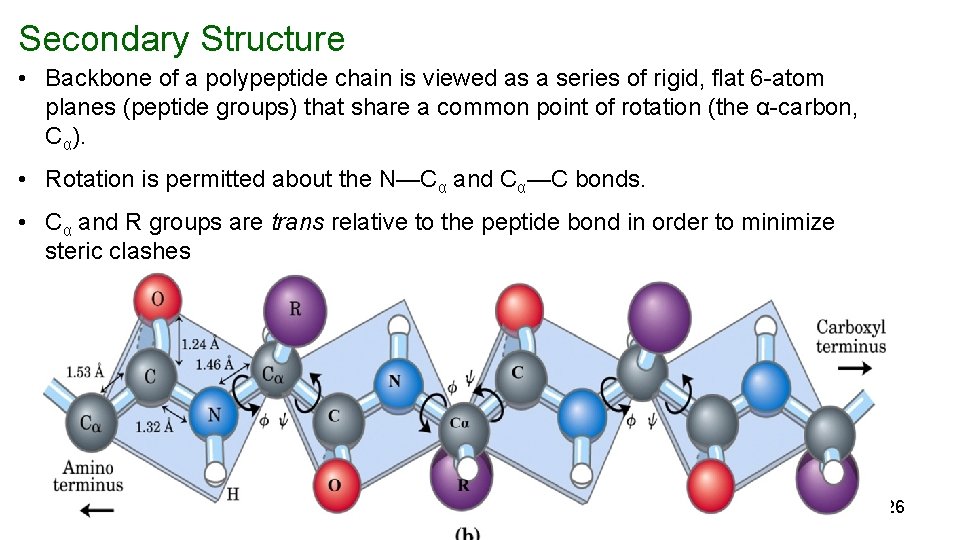
Secondary Structure • Backbone of a polypeptide chain is viewed as a series of rigid, flat 6 -atom planes (peptide groups) that share a common point of rotation (the α-carbon, Cα). • Rotation is permitted about the N—Cα and Cα—C bonds. • Cα and R groups are trans relative to the peptide bond in order to minimize steric clashes 26
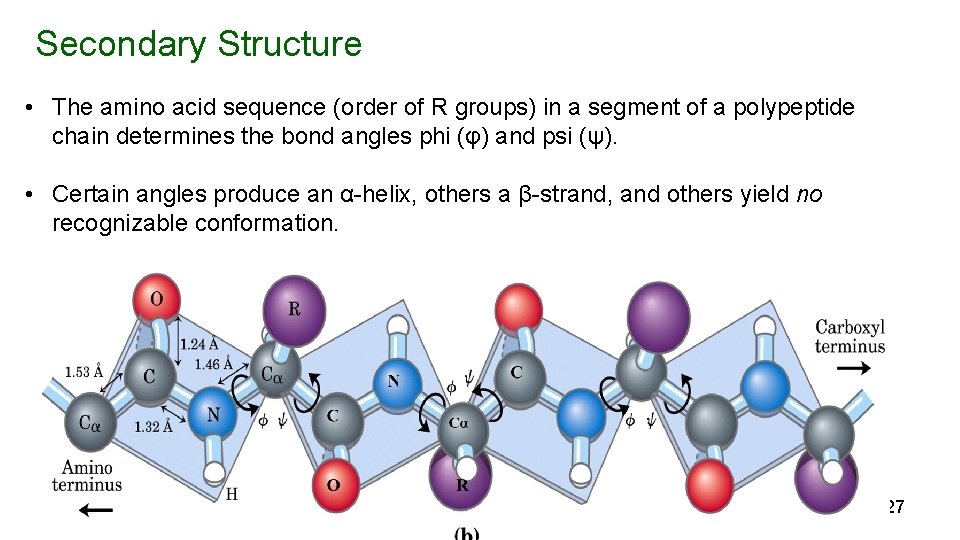
Secondary Structure • The amino acid sequence (order of R groups) in a segment of a polypeptide chain determines the bond angles phi (φ) and psi (ψ). • Certain angles produce an α-helix, others a β-strand, and others yield no recognizable conformation. 27
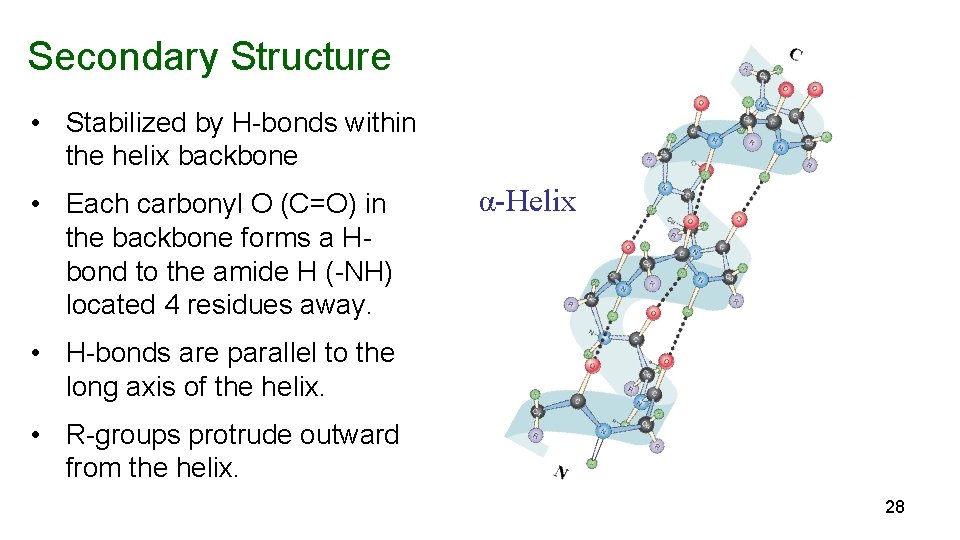
Secondary Structure • Stabilized by H-bonds within the helix backbone • Each carbonyl O (C=O) in the backbone forms a Hbond to the amide H (-NH) located 4 residues away. α-Helix • H-bonds are parallel to the long axis of the helix. • R-groups protrude outward from the helix. 28
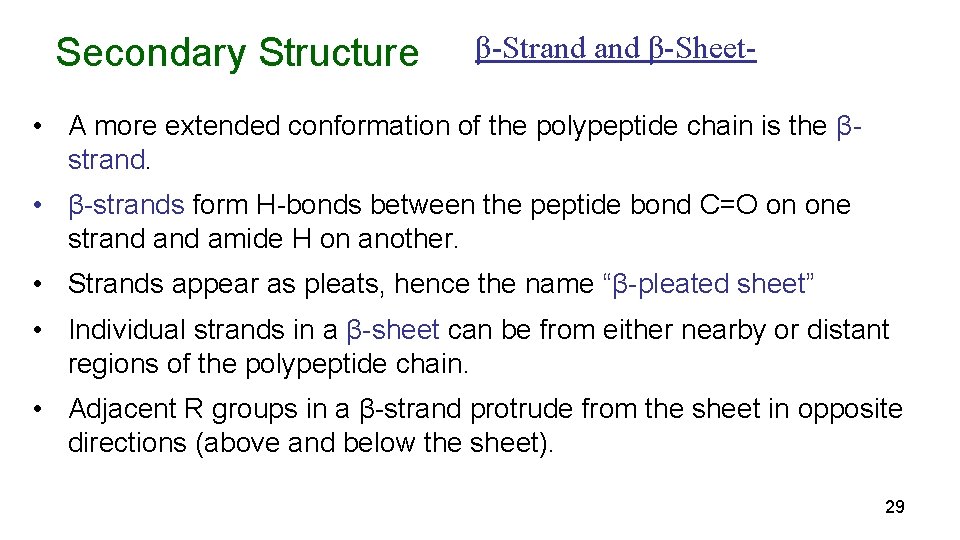
Secondary Structure β-Strand β-Sheet- • A more extended conformation of the polypeptide chain is the βstrand. • β-strands form H-bonds between the peptide bond C=O on one strand amide H on another. • Strands appear as pleats, hence the name “β-pleated sheet” • Individual strands in a β-sheet can be from either nearby or distant regions of the polypeptide chain. • Adjacent R groups in a β-strand protrude from the sheet in opposite directions (above and below the sheet). 29
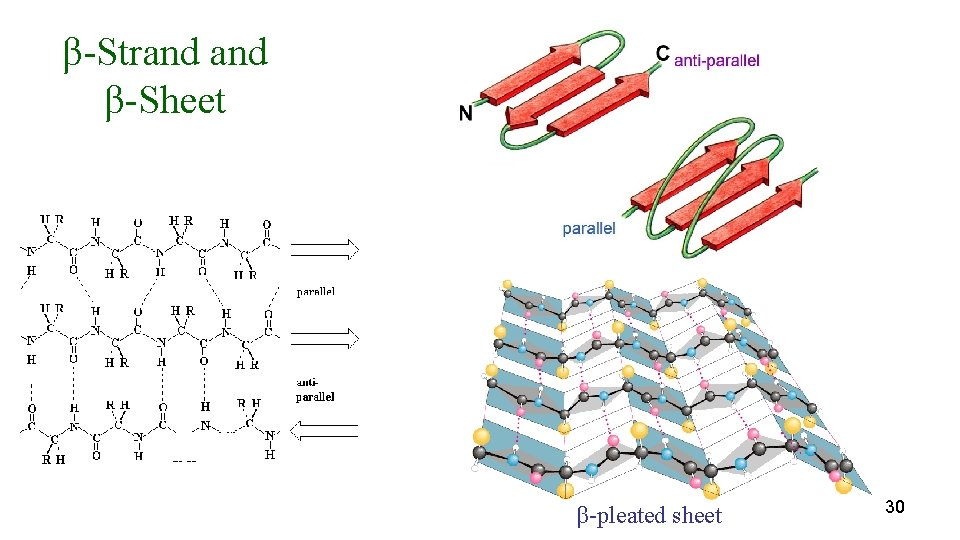
β-Strand β-Sheet β-pleated sheet 30
Tertiary Structure • Tertiary structure is the overall arrangement of the atoms of a protein in 3 -D space. • Many different tertiary structures are possible. • Tertiary structures encompass long-range interactions between the same or different kinds of secondary structural elements. • Amino acids far apart in the primary structure can be close in space in the tertiary structure. 31
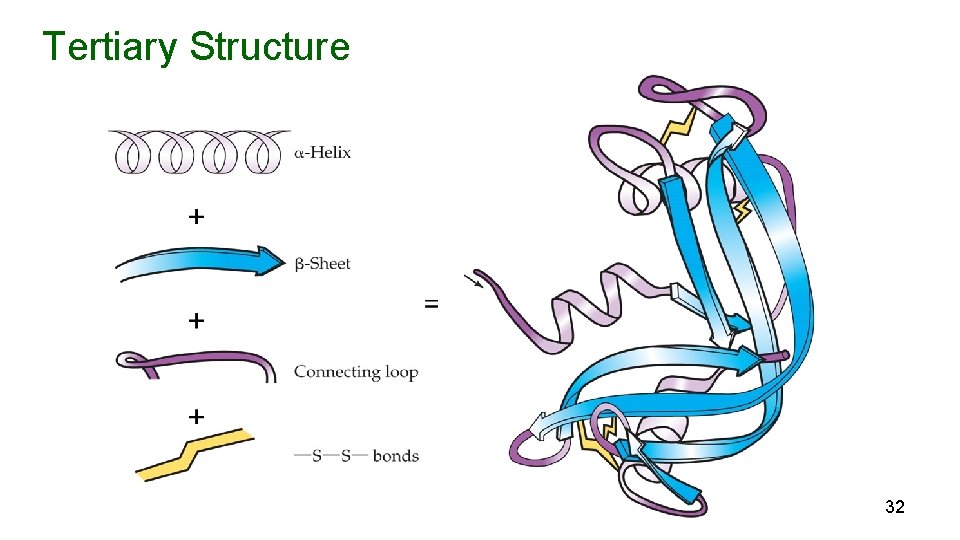
Tertiary Structure + 32
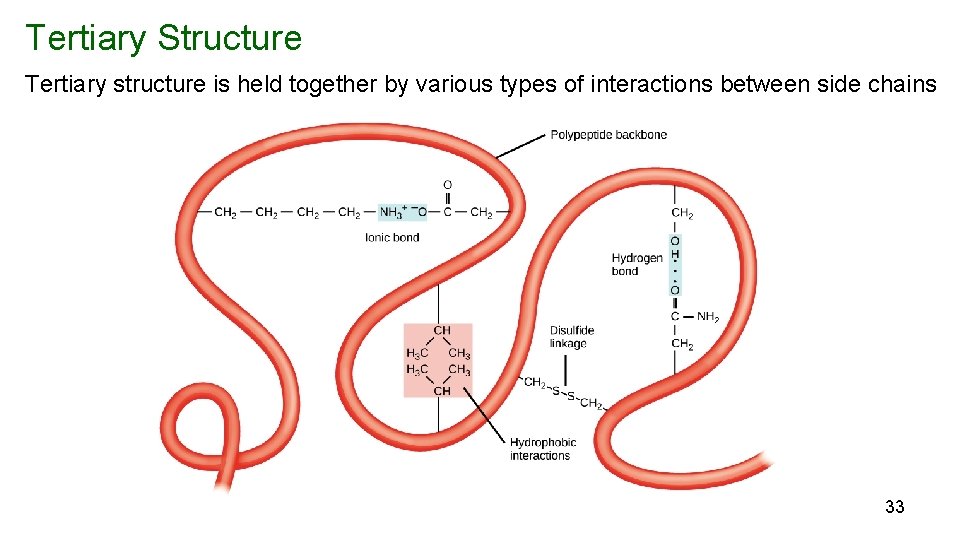
Tertiary Structure Tertiary structure is held together by various types of interactions between side chains 33
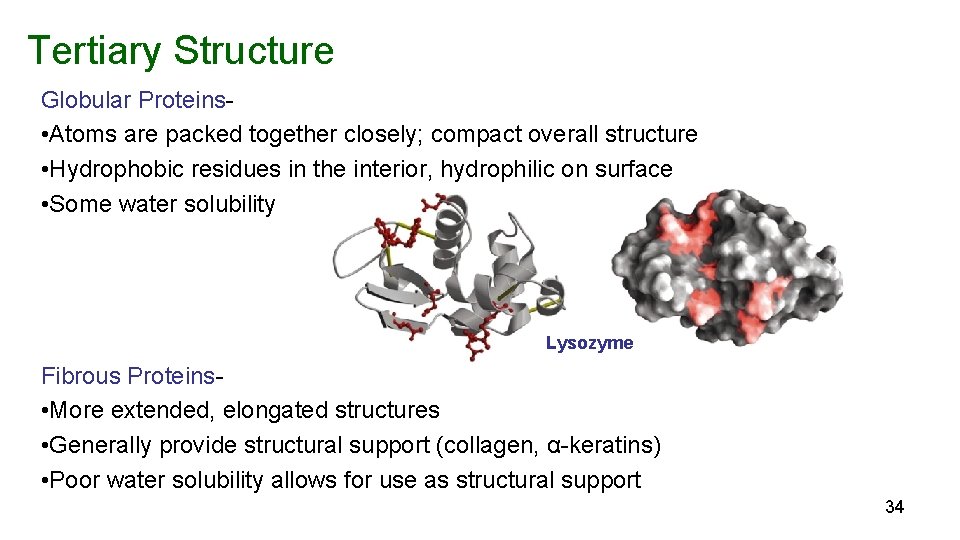
Tertiary Structure Globular Proteins • Atoms are packed together closely; compact overall structure • Hydrophobic residues in the interior, hydrophilic on surface • Some water solubility Lysozyme Fibrous Proteins • More extended, elongated structures • Generally provide structural support (collagen, α-keratins) • Poor water solubility allows for use as structural support 34
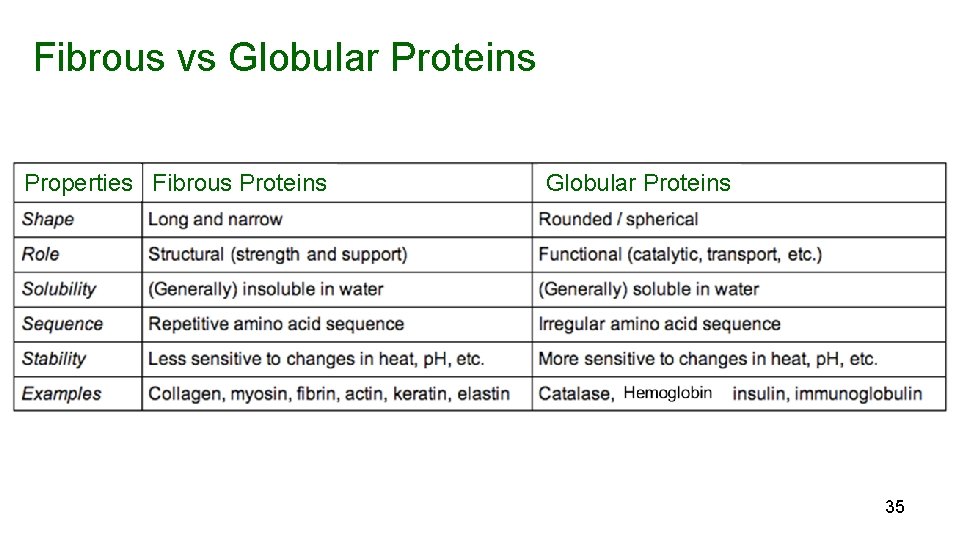
Fibrous vs Globular Proteins Properties Fibrous Proteins Globular Proteins 35
Quaternary Structure Some proteins contain two or more polypeptide chains (subunits) which may be identical or different. Quaternary structure refers to a protein’s subunit structure, i. e how many subunits and how are they are arranged. Subunits may be held together through noncovalent interactions or by disulfide bonds 36
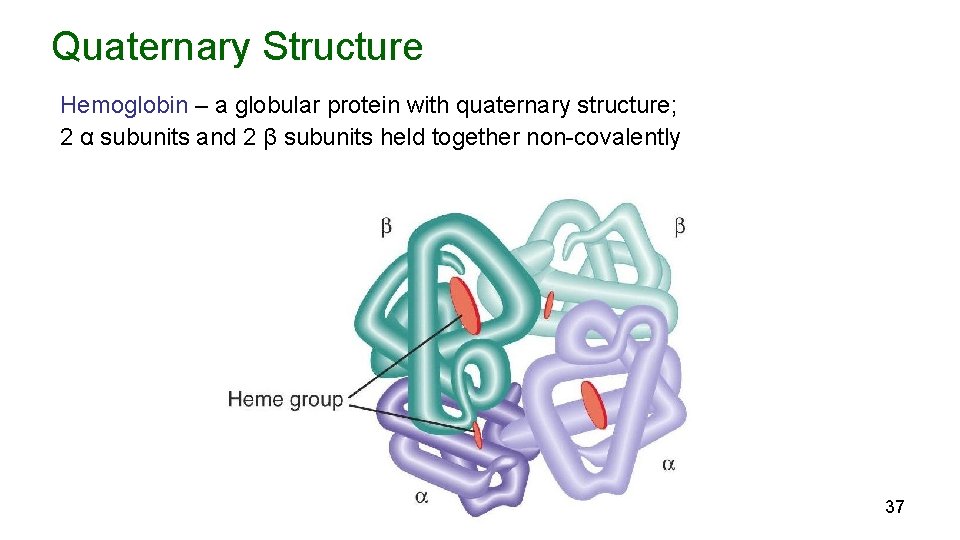
Quaternary Structure Hemoglobin – a globular protein with quaternary structure; 2 α subunits and 2 β subunits held together non-covalently 37
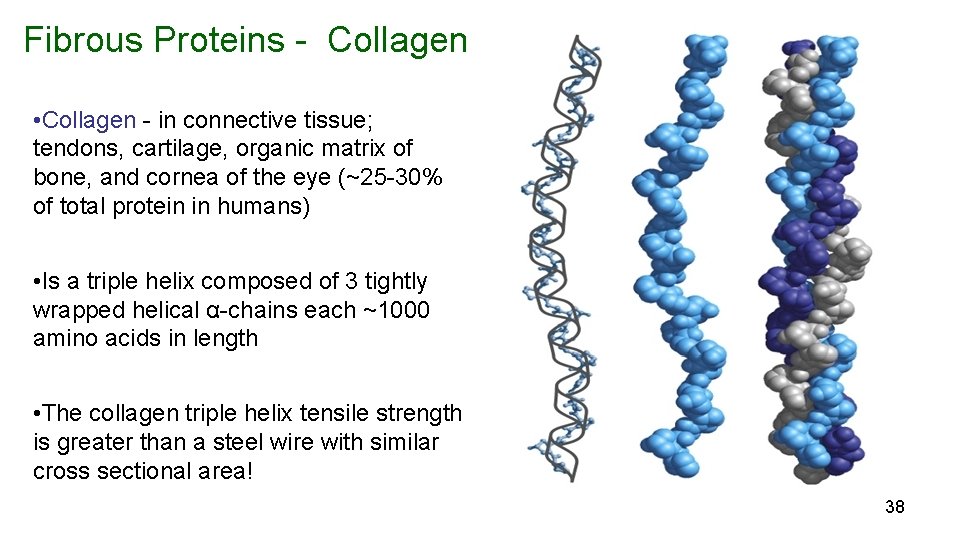
Fibrous Proteins - Collagen • Collagen - in connective tissue; tendons, cartilage, organic matrix of bone, and cornea of the eye (~25 -30% of total protein in humans) • Is a triple helix composed of 3 tightly wrapped helical α-chains each ~1000 amino acids in length • The collagen triple helix tensile strength is greater than a steel wire with similar cross sectional area! 38
- Slides: 38