Module 3 Lesson 1213 Reversible Reactions Dynamic Equilibria

Module 3 Lesson 12/13 Reversible Reactions & Dynamic Equilibria
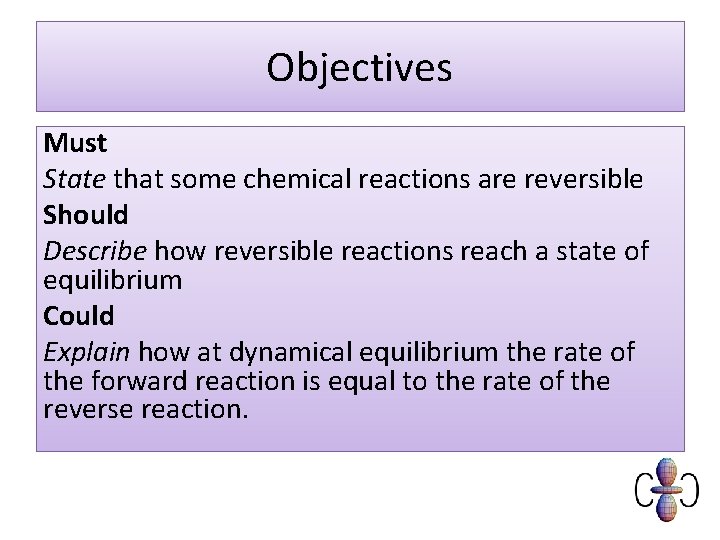
Objectives Must State that some chemical reactions are reversible Should Describe how reversible reactions reach a state of equilibrium Could Explain how at dynamical equilibrium the rate of the forward reaction is equal to the rate of the reverse reaction.

Reversible or not reversible Until now, we were careful to say that most chemical reactions were not reversible – They could not go back to the reactants once the products are formed.
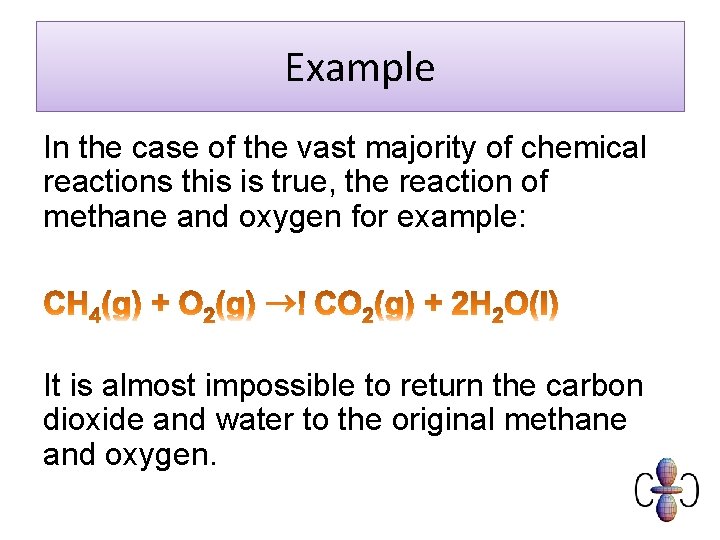
Example In the case of the vast majority of chemical reactions this is true, the reaction of methane and oxygen for example: It is almost impossible to return the carbon dioxide and water to the original methane and oxygen.
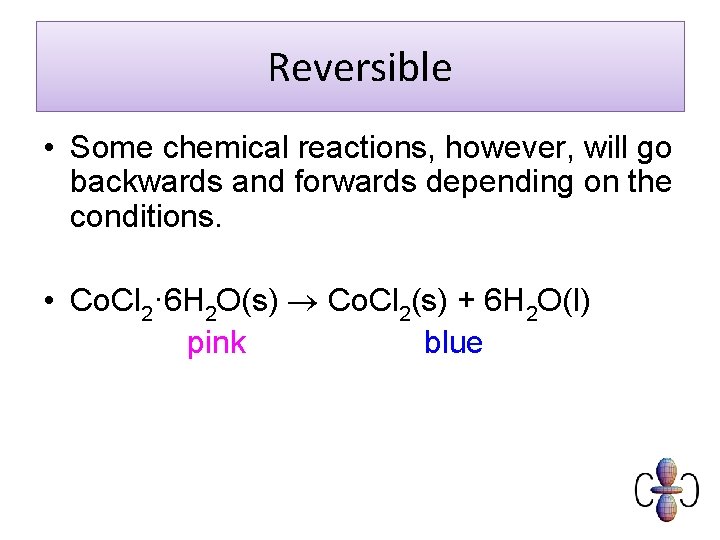
Reversible • Some chemical reactions, however, will go backwards and forwards depending on the conditions. • Co. Cl 2· 6 H 2 O(s) Co. Cl 2(s) + 6 H 2 O(l) pink blue
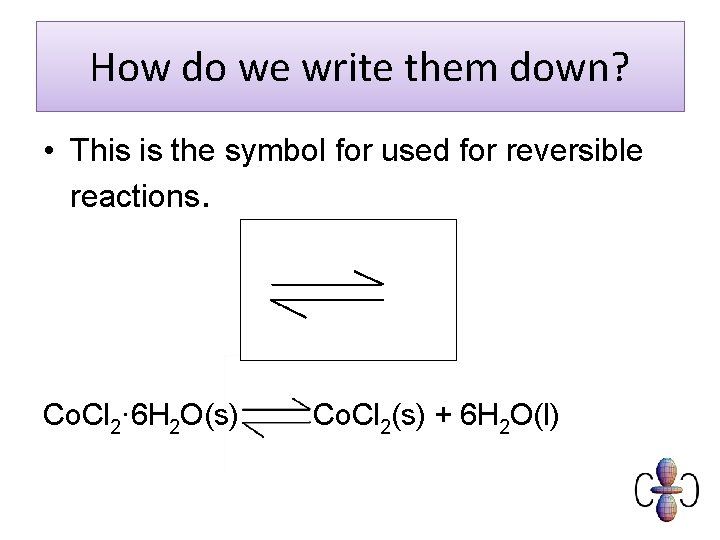
How do we write them down? • This is the symbol for used for reversible reactions. Co. Cl 2· 6 H 2 O(s) Co. Cl 2(s) + 6 H 2 O(l)
What is equilibrium? • Reversible reactions reach a balance point, where the amount of reactants and the amount of products formed remains constant.
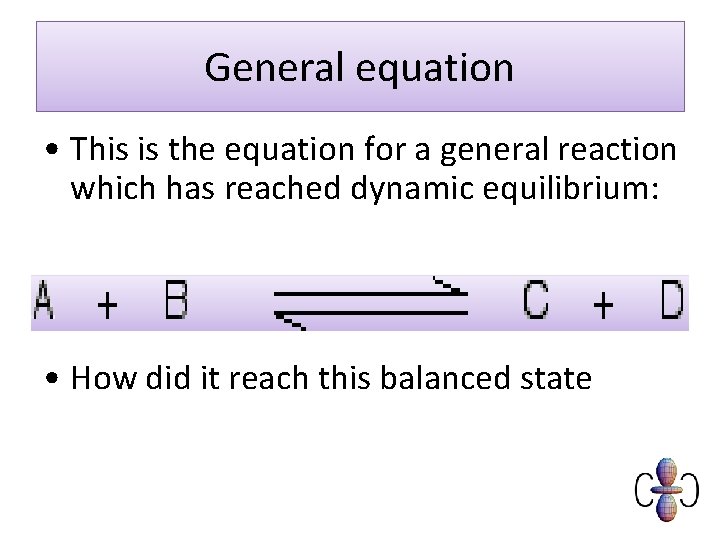
General equation • This is the equation for a general reaction which has reached dynamic equilibrium: • How did it reach this balanced state

• At the beginning of the reaction, the concentrations of A and B were at their maximum. A + B C + D
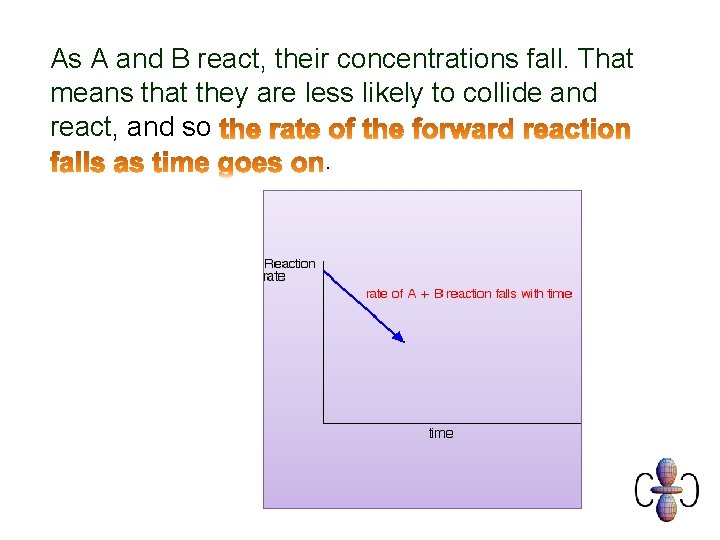
As A and B react, their concentrations fall. That means that they are less likely to collide and react, and so.
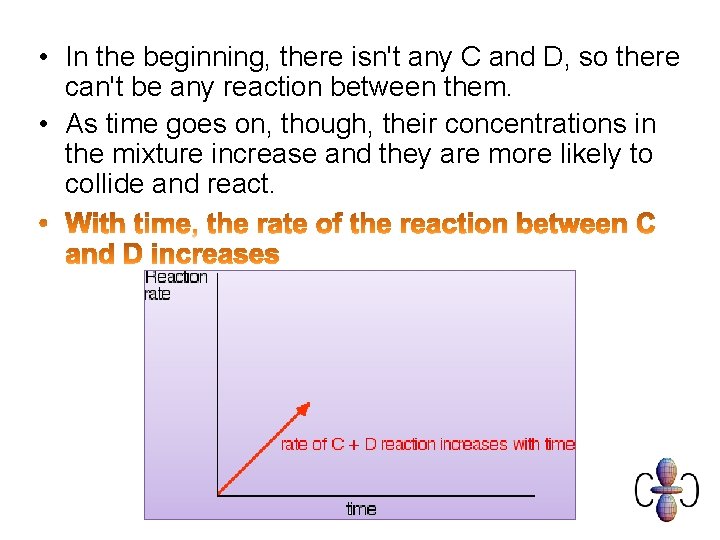
• In the beginning, there isn't any C and D, so there can't be any reaction between them. • As time goes on, though, their concentrations in the mixture increase and they are more likely to collide and react.
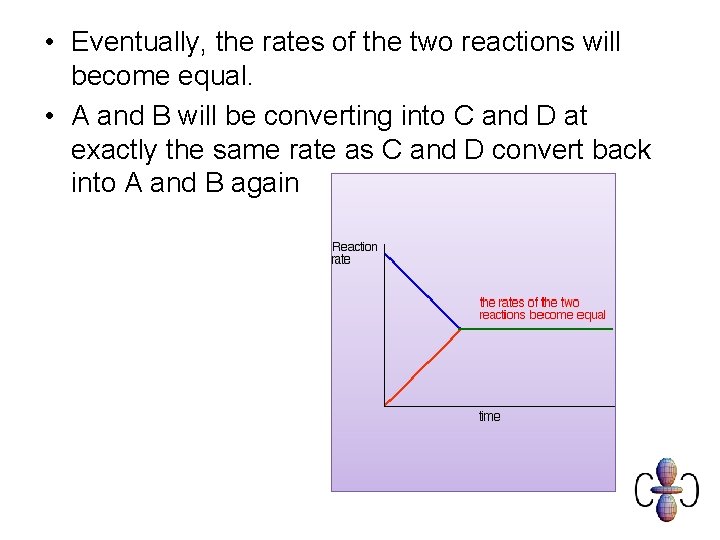
• Eventually, the rates of the two reactions will become equal. • A and B will be converting into C and D at exactly the same rate as C and D convert back into A and B again
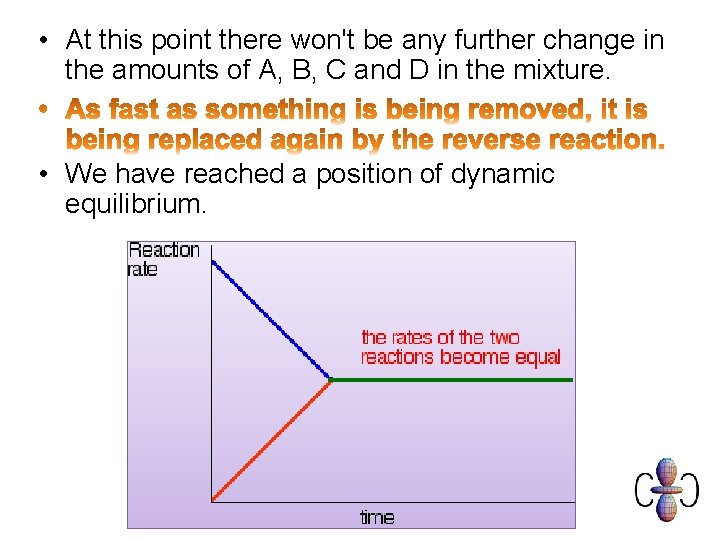
• At this point there won't be any further change in the amounts of A, B, C and D in the mixture. • We have reached a position of dynamic equilibrium.
Dynamic Equilibrium. • In the forward and backwards reactions continue at equal rates so the concentrations of reactants and products do not change. • On a molecular scale there is. • On the macroscopic scale. The system needs to be closed – isolated from the outside world.

Other examples 2 NO 2 N 2 O 4 Briggs Rauscher – an awesome set of 10 competing chemical reactions in equilibrium

Plenary - Examination questions • As a warm up we will use some GCSE level answers.
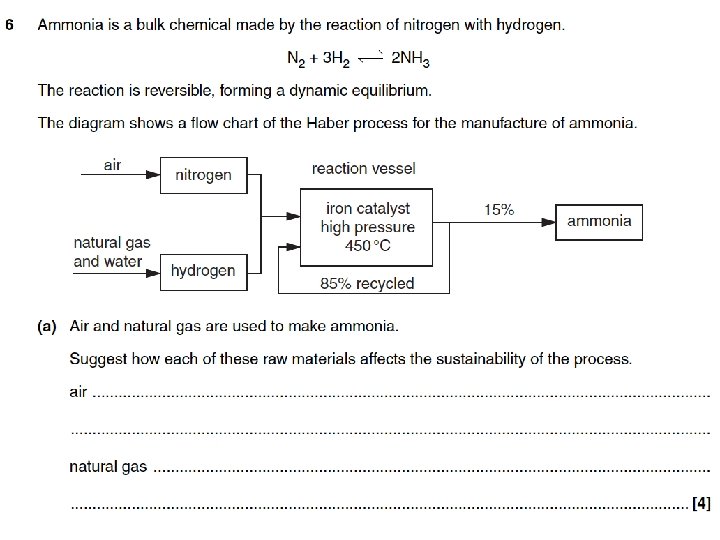
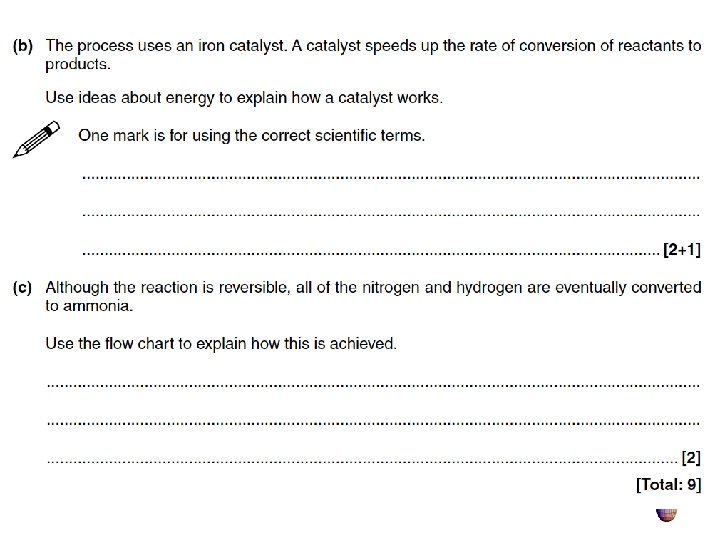
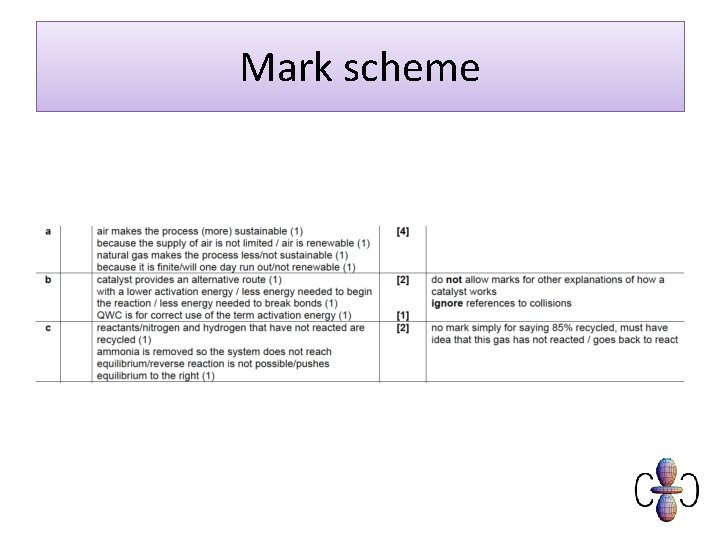
Mark scheme
Summary State that some chemical reactions are reversible Describe how reversible reactions reach a state of equilibrium Explain this using dynamic equilibrium model.
- Slides: 20