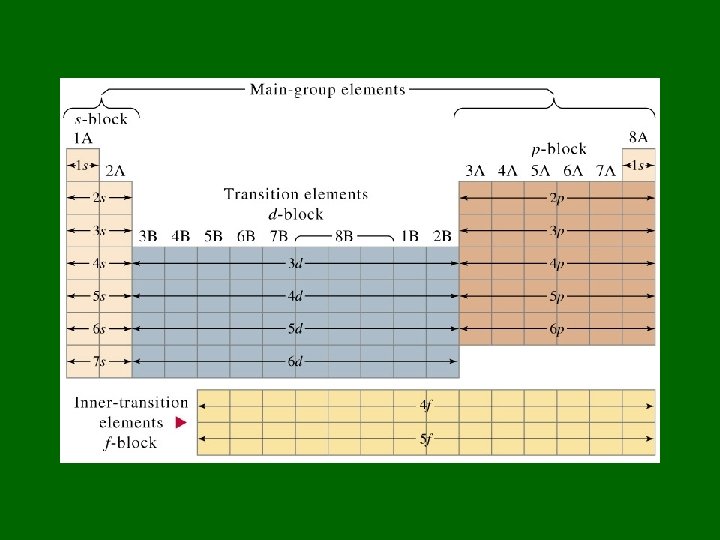
Modern Theory of the Atom Quantum Mechanical Model













- Slides: 39

Modern Theory of the Atom Quantum Mechanical Model Or Wave Mechanical Model Or Schrodinger’s Model

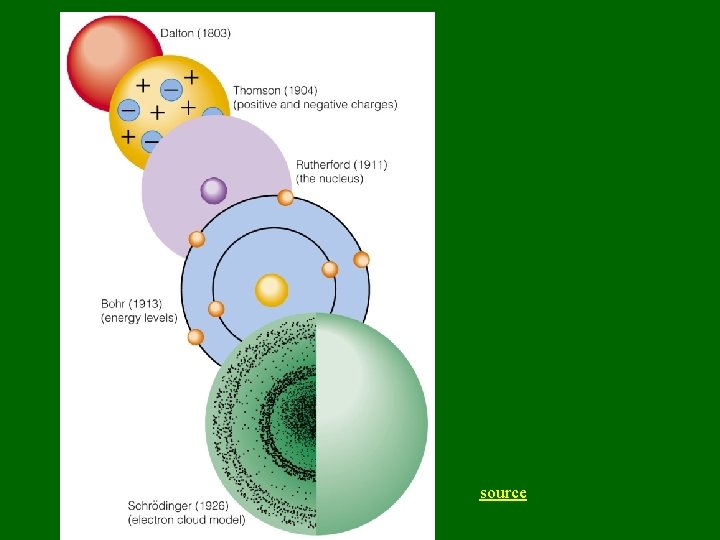
source

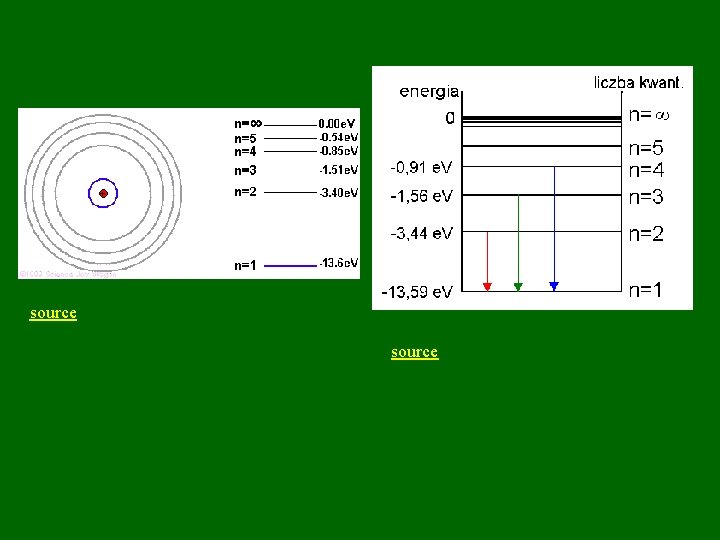
Recap of Bohr Model • Electrons treated as particles moving in circular orbits. Specify speed, position, energy. • Quantization of energy levels is imposed. • Ground state: electrons close to nucleus • Electron transitions between energy levels can occur. Higher energy levels are farther from nucleus. – Moving up, electron absorbs energy – Moving down, electron emits light energy • Wavelengths of light in H spectrum can be predicted. Depend on energy difference of 2 levels involved in transition.

source

1924: De Broglie • Proposed that if light can show both particle and wave behavior, maybe matter can too.



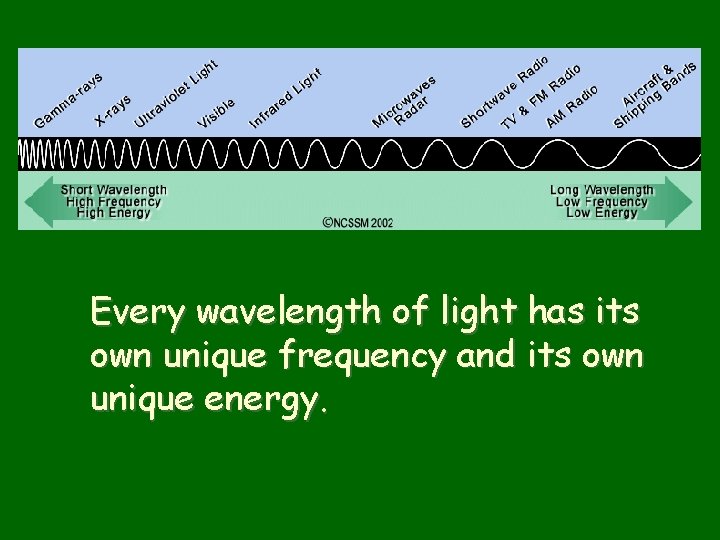
Every wavelength of light has its own unique frequency and its own unique energy.

2 kinds of waves Traveling wave • Wave is not confined to a given space • Travels from one location to another • Interrupted by a boundary or another wave Standing wave • Confined to a given space. (Ends pinned. ) • Interference between incident & reflected waves. • At certain frequencies, certain points seem to be standing still. • Other points, displacement changes in a regular way.

Traveling Wave #1 • Traveling Wave #2

Guitar string • Standing wave #1

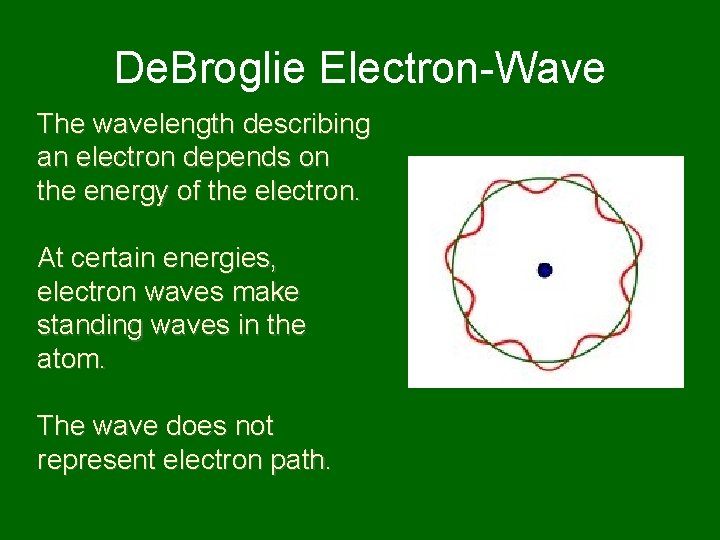
De. Broglie Electron-Wave The wavelength describing an electron depends on the energy of the electron. At certain energies, electron waves make standing waves in the atom. The wave does not represent electron path.

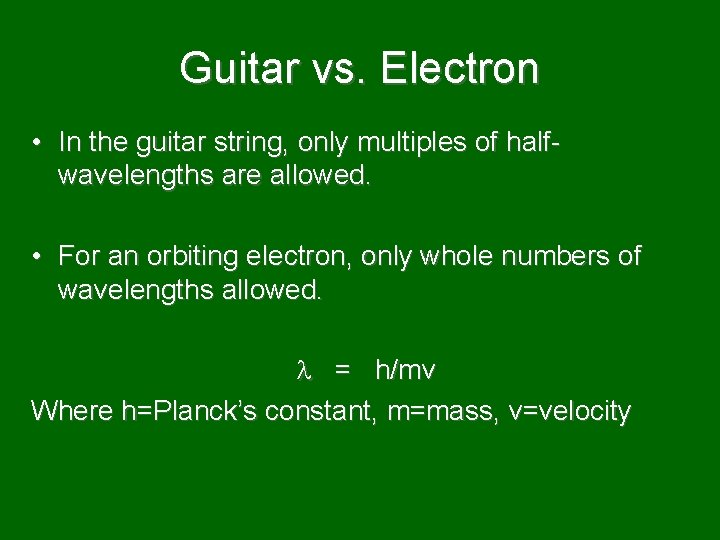
Guitar vs. Electron • In the guitar string, only multiples of halfwavelengths are allowed. • For an orbiting electron, only whole numbers of wavelengths allowed. = h/mv Where h=Planck’s constant, m=mass, v=velocity

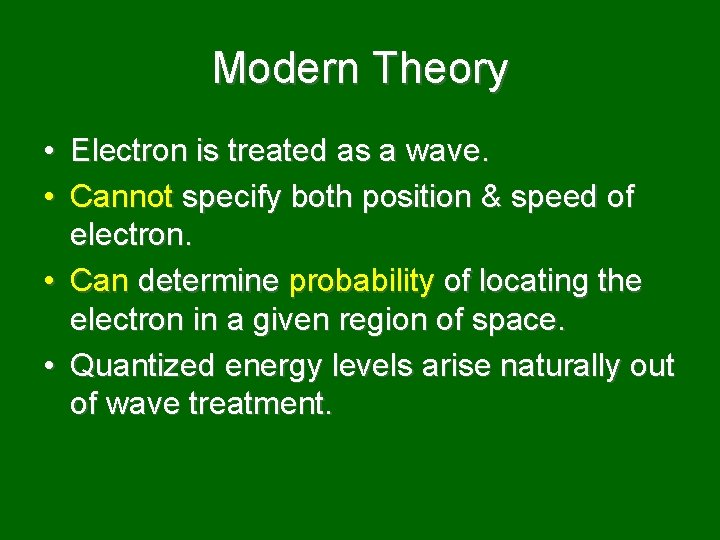
Modern Theory • Electron is treated as a wave. • Cannot specify both position & speed of electron. • Can determine probability of locating the electron in a given region of space. • Quantized energy levels arise naturally out of wave treatment.

Heisenberg uncertainty principle • Fundamentally impossible to know the velocity and position of a particle at the same time. • Impossible to make an observation without influencing the system.

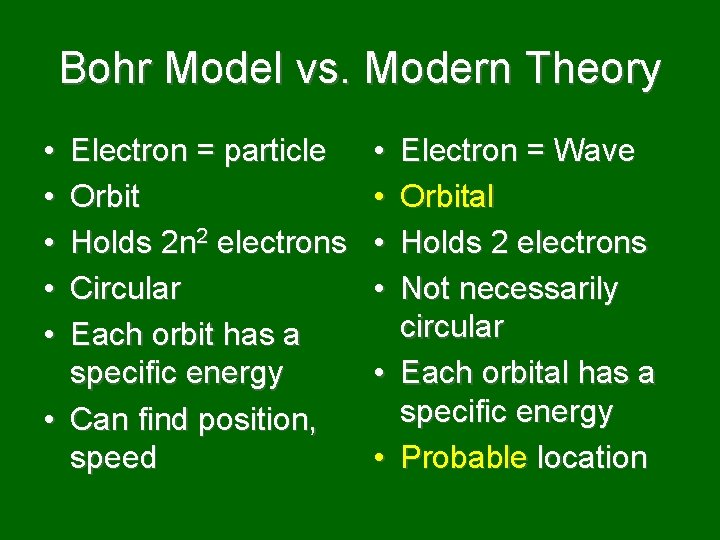
Bohr Model vs. Modern Theory • • • Electron = particle Orbit Holds 2 n 2 electrons Circular Each orbit has a specific energy • Can find position, speed • • • Electron = Wave Orbital Holds 2 electrons Not necessarily circular Each orbital has a specific energy Probable location

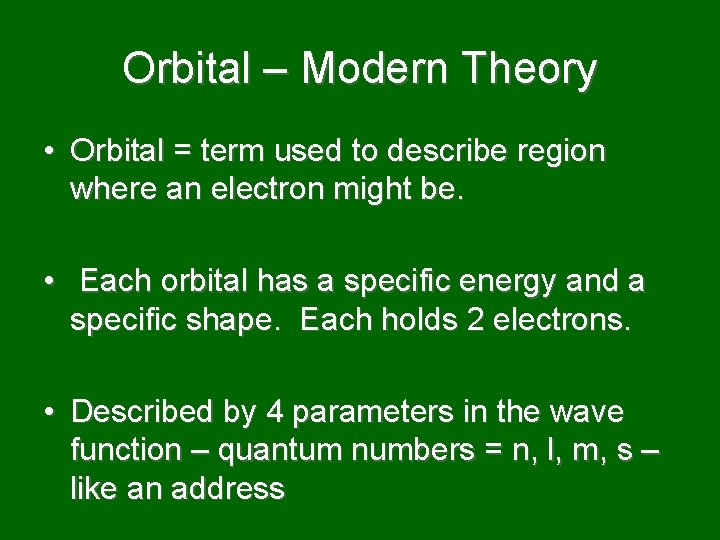
Orbital – Modern Theory • Orbital = term used to describe region where an electron might be. • Each orbital has a specific energy and a specific shape. Each holds 2 electrons. • Described by 4 parameters in the wave function – quantum numbers = n, l, m, s – like an address

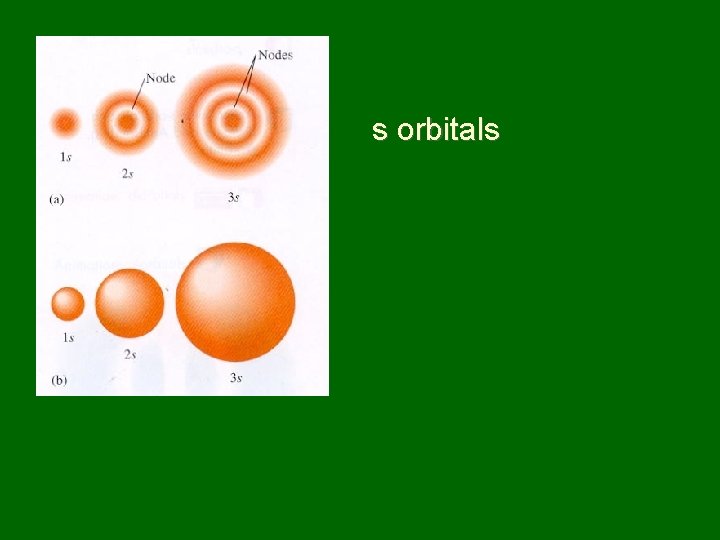
s orbitals

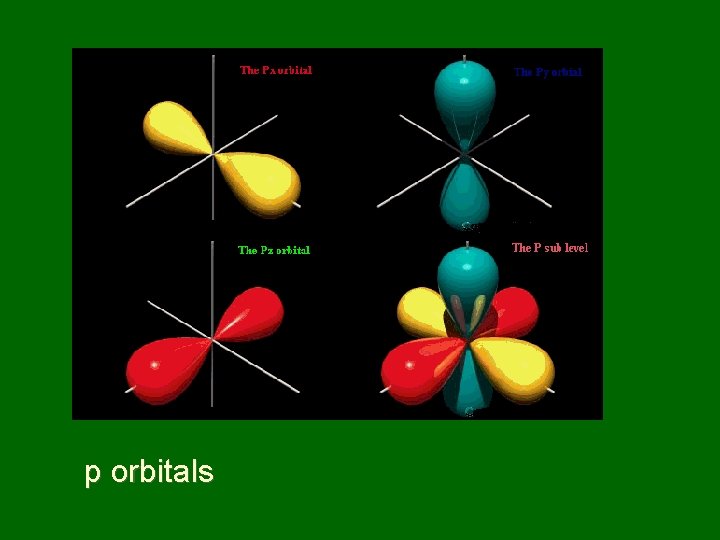
p orbitals

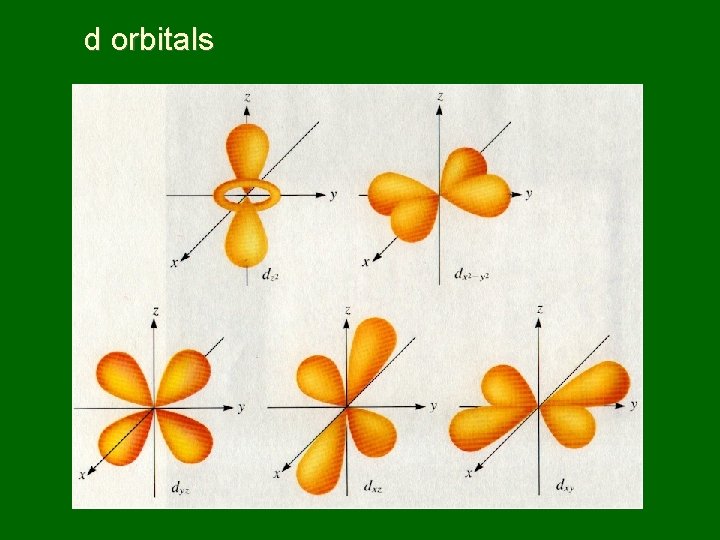
d orbitals

What can orbitals do for us? • Physical structure of orbitals explains – Bonding – Magnetism – Size of atoms – Structure of crystals

Quantum Numbers • Each electron in an atom has a set of 4 quantum numbers – like an address. • No two electrons can have all 4 quantum numbers the same. – n = principal energy level, n = 1, 2, 3, 4, . . . – l = type of orbital, l= 0, 1, 2, 3, n-1 – ml = orientation of orbital, ml = -l, …, 0, … +l – s or ms = electron spin = +1/2 or -1/2

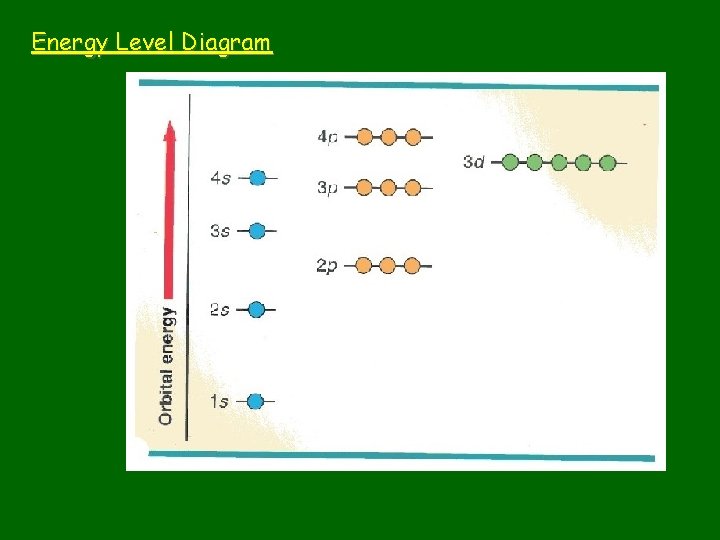
Energy Level Diagram

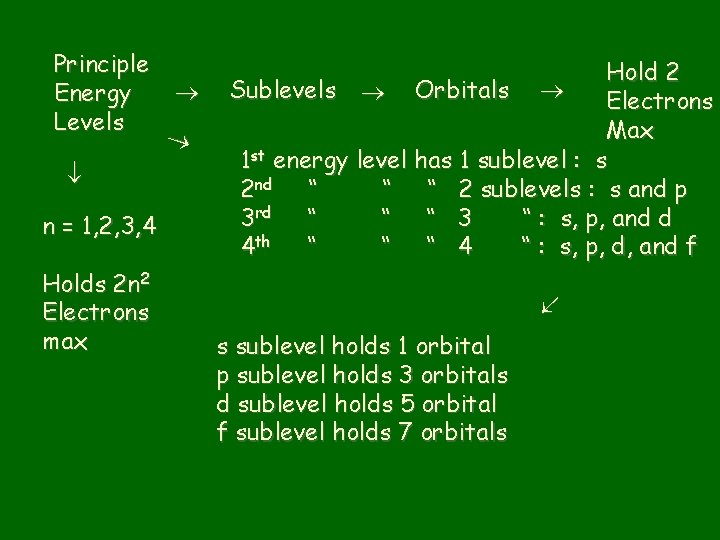
n: principal quantum number • Specifies atom’s major (principal) energy levels • Has whole number values: 1, 2, 3, 4, … • Maximum # of electrons in any principal energy level = 2 n 2

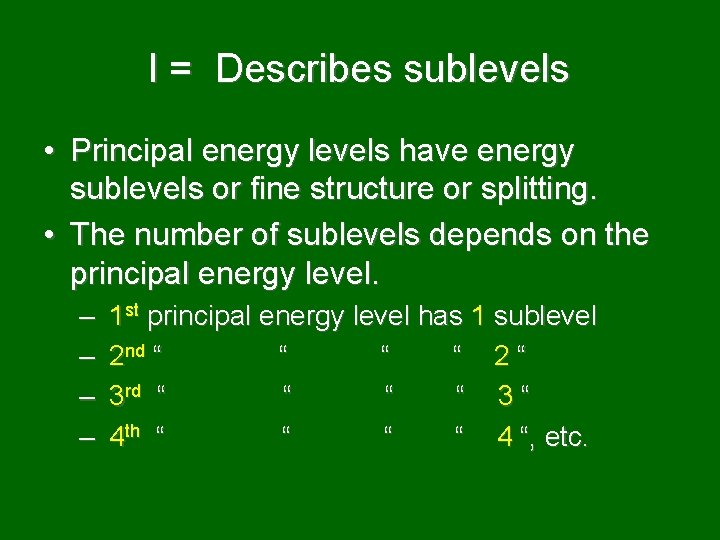
l = Describes sublevels • Principal energy levels have energy sublevels or fine structure or splitting. • The number of sublevels depends on the principal energy level. – – 1 st principal energy level has 1 sublevel 2 nd “ “ 2“ 3 rd “ “ 3“ 4 th “ “ 4 “, etc.

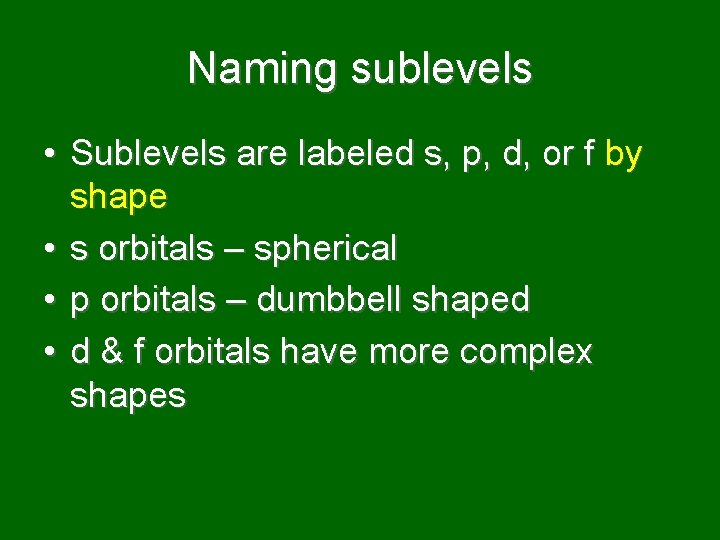
Naming sublevels • Sublevels are labeled s, p, d, or f by shape • s orbitals – spherical • p orbitals – dumbbell shaped • d & f orbitals have more complex shapes

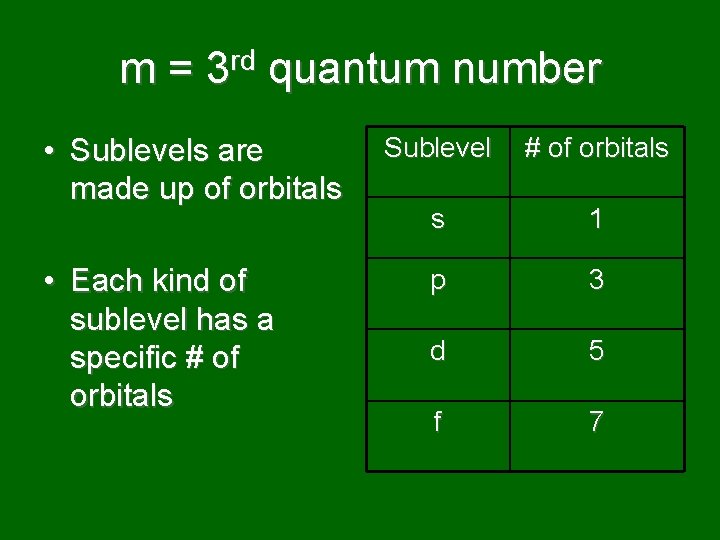
m = 3 rd quantum number • Sublevels are made up of orbitals • Each kind of sublevel has a specific # of orbitals Sublevel # of orbitals s 1 p 3 d 5 f 7

4 th quantum number = s • Electron spin - 2 possible values • 4 quantum numbers = address for each electron. • No 2 electrons in an atom can have the same 4 quantum numbers. Thus, only 2 electrons per orbital. • Pauli exclusion principle.

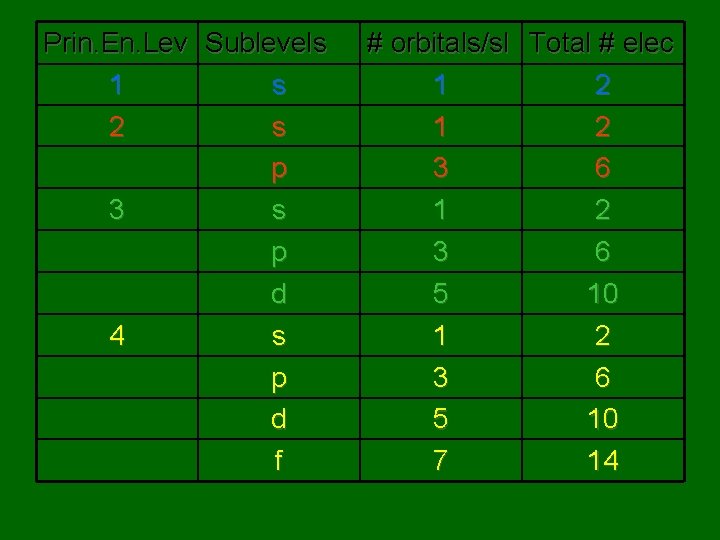
Prin. En. Lev Sublevels 1 s 2 s p 3 s p d 4 s p d f # orbitals/sl Total # elec 1 2 3 6 5 10 7 14

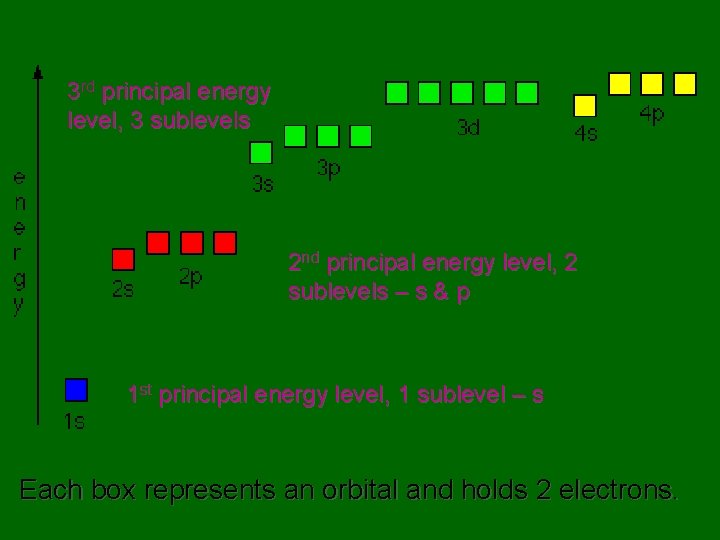
3 rd principal energy level, 3 sublevels 2 nd principal energy level, 2 sublevels – s & p 1 st principal energy level, 1 sublevel – s Each box represents an orbital and holds 2 electrons.

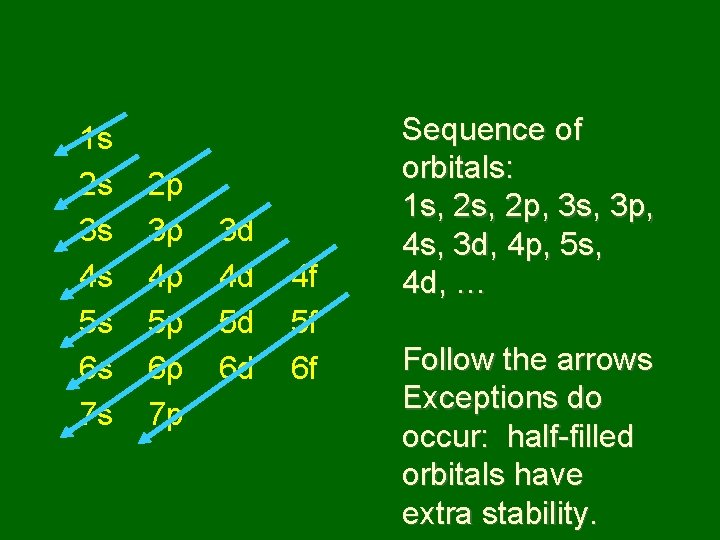
Order of fill: Aufbau principle • Each electron occupies the lowest orbital available • Learn sequence of orbitals from lowest to highest energy • Is some overlap between sublevels of different principal energy levels

1 s 2 s 3 s 4 s 5 s 6 s 7 s 2 p 3 p 4 p 5 p 6 p 7 p 3 d 4 d 5 d 6 d 4 f 5 f 6 f Sequence of orbitals: 1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s, 4 d, … Follow the arrows Exceptions do occur: half-filled orbitals have extra stability.

Hund’s Rule • Distribution of electrons in equal energy orbitals: Spread them out as much as possible!

Electron Configurations

Compare Bohr & Schrodinger

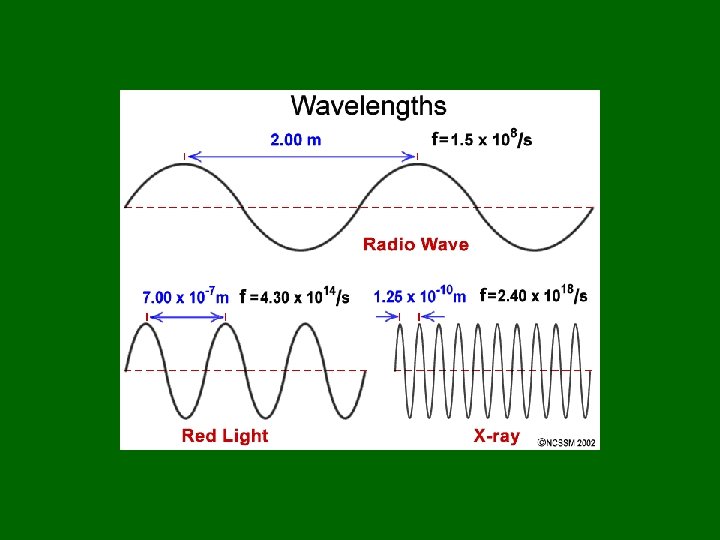
Frequencies in Chemistry

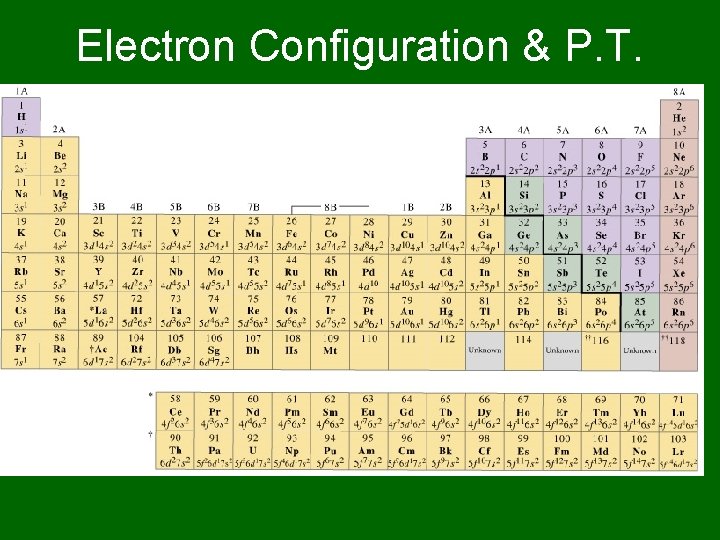
Electron Configuration & P. T.


n = 1, 2, 3, 4 Hold 2 Sublevels Orbitals Electrons Max 1 st energy level has 1 sublevel : s 2 nd “ “ “ 2 sublevels : s and p 3 rd “ “ “ 3 “ : s, p, and d 4 th “ “ “ 4 “ : s, p, d, and f Holds 2 n 2 Electrons max Principle Energy Levels s sublevel holds 1 orbital p sublevel holds 3 orbitals d sublevel holds 5 orbital f sublevel holds 7 orbitals