Modern Physics NCEA AS 3 5 Text Chapters























































- Slides: 76

Modern Physics NCEA AS 3. 5 Text Chapters: 20, 22

The Photoelectric Effect The photoelectric effect occurs when shining light (usually UV) onto a piece of metal causes electrons to be given off. l This effect can be used in a photoelectric cell to produce small electric currents. l Photoelectric cells are used in l l Light meters l Burglar alarms l TV cameras etc

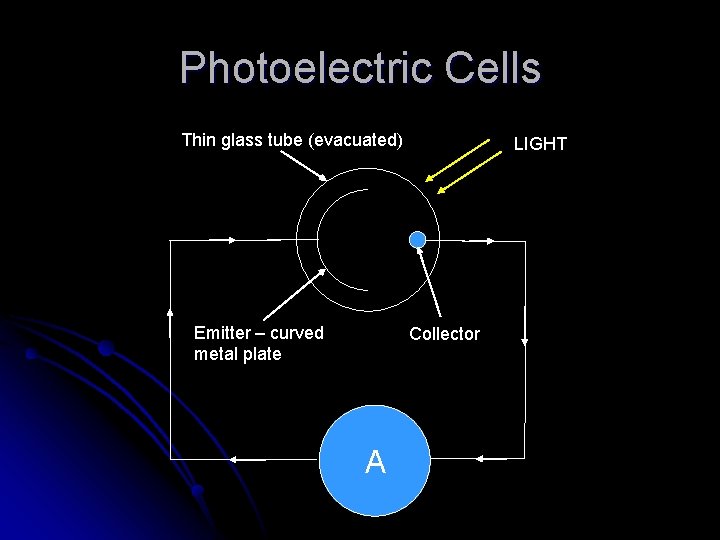
Photoelectric Cells Thin glass tube (evacuated) Emitter – curved metal plate LIGHT Collector A

Photoelectric Experiments When the photoelectric effect was studied in detail, the experimental results were very different to what was expected. A new theory about the nature of light was needed to explain what happened. l Scientists at the time considered light to behave like a wave…… l

Photoelectric Experiments l What was expected: l Brighter light would cause electrons with more kinetic energy to be emitted l But what actually happened?

Photoelectric Experiments l. Brighter light caused more electrons to be emitted, but there was no change in the amount of energy they had. l. What l. If was expected: very dim light was used, it would take some time before any electrons had absorbed enough energy to escape from the metal

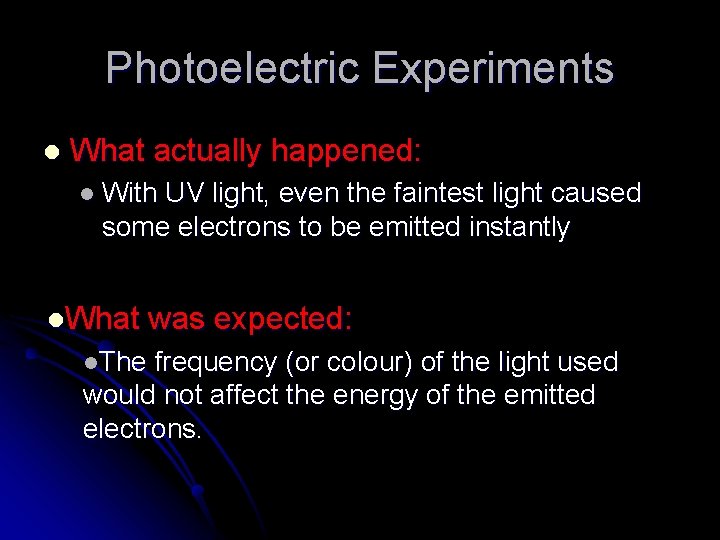
Photoelectric Experiments l What actually happened: l With UV light, even the faintest light caused some electrons to be emitted instantly l. What l. The was expected: frequency (or colour) of the light used would not affect the energy of the emitted electrons.

Photoelectric Experiments l What actually happened: l The higher the frequency, the higher the energy of the electrons. Below a certain frequency, no electrons were emitted.

What did you take in? Try and answer these without looking back at yesterdays notes 1) What is a photo electron? 2) Which theory of light does the photo electric effect support? 3) What quantity dictates the amount of energy a photo electron contains? 4) What is an electron-volt? 5) Does dull light produce photo-electrons? What happens when the same light is brightened up? All type 1 questions

Usually 2 achieved type 1 ticks are given away per paper for knowing the correct units and significant figures Imagine being called Plank? l l l What are the units for magnetic flux? …………………… impulse? …………………… rotational inertia? 3. 00 x 108 how many s. f. ? 0. 003450 how many s. f. ? Some of you could have passed with 2 extra type 1 marks so get into the habit of using the correct units and s. f. s

Photoelectric Experiments Einstein explained these results, using an idea suggested by Max Planck, that said electromagnetic radiation comes in fixed “packets” or quanta of energy called photons l The amount of energy each photon has depends on the frequency of the radiation. l

Photoelectric Experiments l Each photon has a fixed amount of energy given by: h=Planck’s Constant = 6. 63 x 10 -34 Js l This suggested that light behaved like a moving particle, rather than a wave l

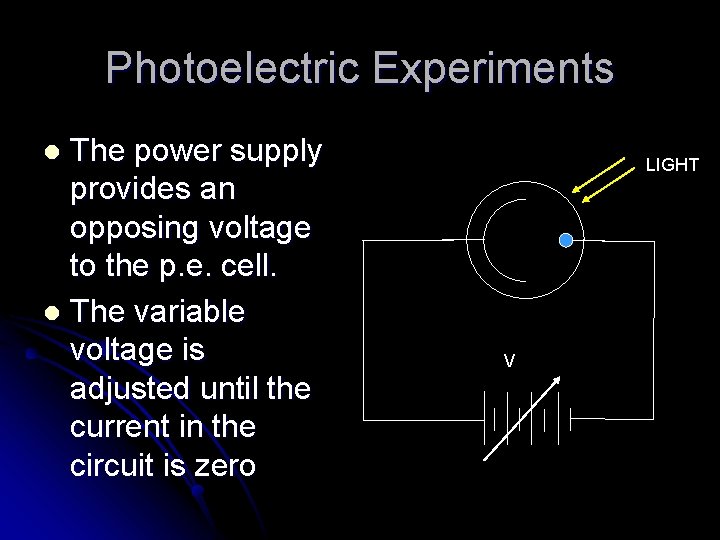
Photoelectric Experiments The power supply provides an opposing voltage to the p. e. cell. l The variable voltage is adjusted until the current in the circuit is zero l LIGHT V

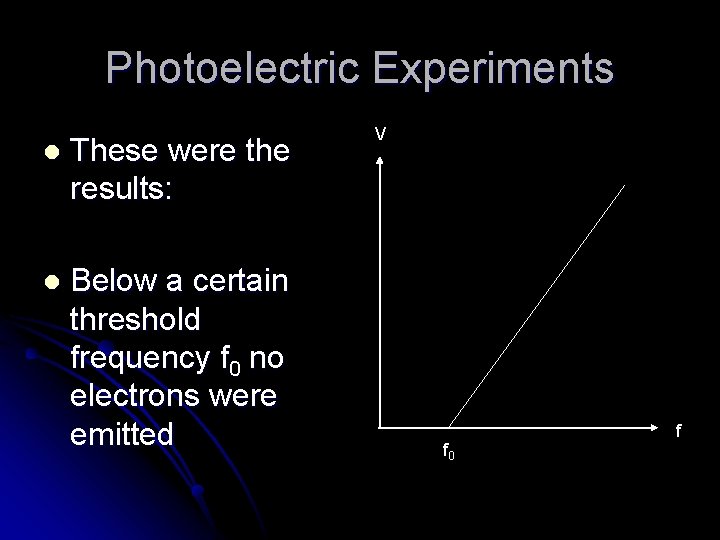
Photoelectric Experiments When the current was zero, the supply voltage was equal to the cut-off voltage of the cell l Different frequencies of light were tried, and the cut-off voltages measured: l

Photoelectric Experiments l These were the results: l Below a certain threshold frequency f 0 no electrons were emitted V f 0 f

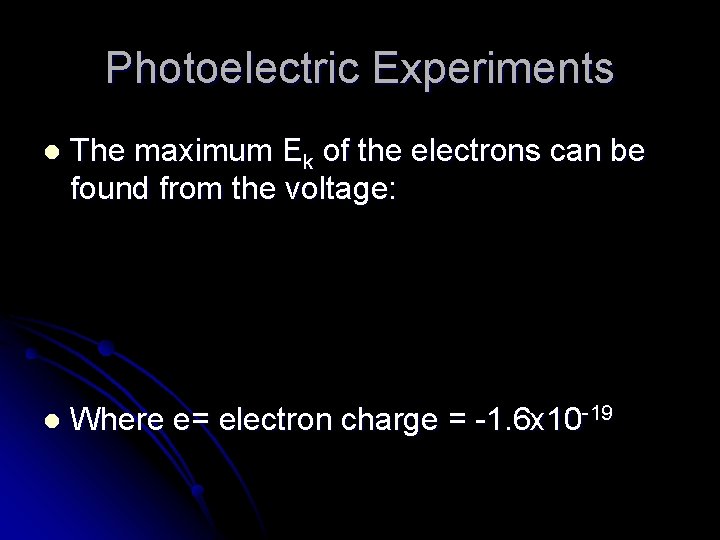
Photoelectric Experiments l The maximum Ek of the electrons can be found from the voltage: l Where e= electron charge = -1. 6 x 10 -19

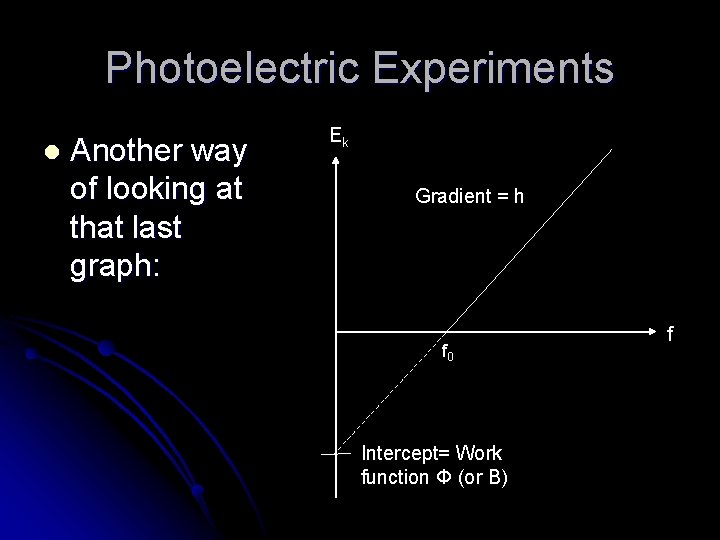
Photoelectric Experiments l Another way of looking at that last graph: Ek Gradient = h f 0 Intercept= Work function Φ (or B) f

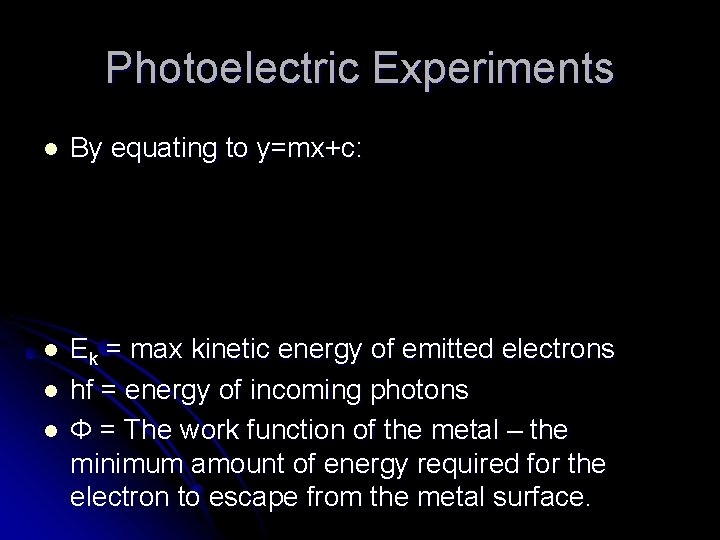
Photoelectric Experiments l By equating to y=mx+c: l Ek = max kinetic energy of emitted electrons hf = energy of incoming photons Φ = The work function of the metal – the minimum amount of energy required for the electron to escape from the metal surface. l l

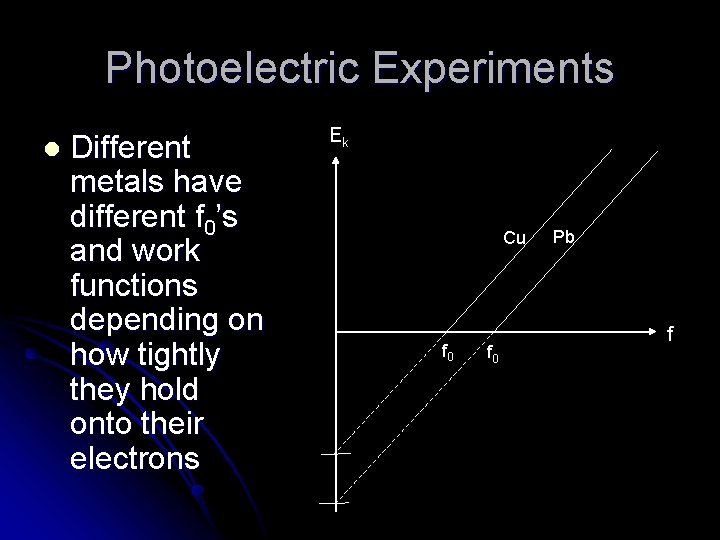
Photoelectric Experiments l Different metals have different f 0’s and work functions depending on how tightly they hold onto their electrons Ek Cu f 0 Pb f

The Conclusion So the photoelectric effect could be explained by thinking of light as a stream of incoming particles that collided with electrons in the metal. If the photon had enough energy, it could knock the electron free of the metal and send it across the cell to the collector. l If photon was too small, it couldn’t hit electrons hard enough (overcome work function) so no electrons emitted. l

Type 1 training – Part 2 It is important that you are able to explain what relatively straight forward words and concepts What do the following mean? (don’t yell out – write it down) õ Capacitor An electrical device that stores charge õ Transverse wave A wave where the particles move at right angles to the wave direction õ Angular Momentum is conserved The angular momentum of a system will remain the same provided there is no net external torque.

Some More õElectric field A region of space in which a charged particle experiences a force. õResonance When the frequency of a forced motion like SHM equals a natural frequency, resulting in a large amplitude. õDoppler Effect A change in observed frequency caused by relative motion between the observor and source of the waves.

Task to do at home. Make a spelling list of physics words, and terms. Eg. Simple Harmonic Motion, standing waves, path difference ……………. . Then see how many you can write a proper definition for. Check the answers with the glossary in the back of your text. Do it tonight and again a few times before the 20 th November.

More energy is required to remove an electron from iron than from calcium. Which of the metals has a higher work function? Iron Which of the metals has a higher threshold frequency? Iron

A beam of 640 nm red light shines onto a polished copper disc. 1. Calculate the energy of each photon E=hf=hc/ =3. 11 x 10 -19 J 2. Write this energy in e. V 1 e. V=1. 6 x 10 -19 J 1. 9 e. V The work function of copper is 1. 28 x 10 -18 J 3. Figure out whether an electron will be emitted That’s 12. 8 x 10 -19 J. 3. 11 x 10 -19 J is about ¼ of what's needed NO 4. What about 160 nm light? 12. 4 x 10 -19 J. Still not quiet enough 5. What is 160 nm Light? UV

Atomic Spectra l The spectral lines are caused by the movement of electrons between different energy shells in the atom

Atomic Spectra l 2 types l Emission – certain frequencies of light given off by low pressure gases excited by heat or electricity l Absorption – certain frequencies absorbed from a continuous spectrum by low pressure gases l Spectra are unique to each element and can be used to identify unknown elements

Type 1 training – Part 3 You have to be able to draw fully label accurate diagrams õDraw a force diagram of an aeroplane (1500 kg) banked at 450. Show components of the lift force. õDraw a labeled phasor diagram and graph for a SHM. Y=-3. 0 sin 0. 31 t õAdd the phasors for velocity and acceleration õDraw a diagram of an ambo’s siren sound waves as it’s speeds along the street.

Another One õ A laser is shone through a double slit onto a screen. Then the double slit is replaced with a diffraction grating of many slits. Draw two labeled diagrams to show the different patterns produced.

Type 1 training – Last Part The last and most common type 1 question is figuring out and explaining a physics situation. Discuss what happens when(don’t yell out – write it down) õ Two cars of very similar mass and speed hit head on. õ Current is being passed through an LED incorrectly. õ A flat stone hits the surface of a smooth lake at a very small angle to the water, traveling reasonably fast

A Few More õA spring has a very large spring constant õYour radio when in behind a hill only receives an AM signal and not FM

Try This The diagram above shows the mode of vibration of the string. Describe the type of wave formed on the guitar string. Stationary wave; transverse and 1 Standing fundamental of first harmonic wave. allowed. Longitudinal or higher harmonics disallowed.

A Humdinger õThe frequency of the note produced by a guitar string is usually altered by changing the length of the string that vibrates. State two other factors that can alter the frequency of notes produced by guitar strings. Explain how they affect the frequency

Factors: 1. Changing the heaviness (mass per meter) of the string. 2. Increase the tension of the string. 1 Correct factors

Factors: 1. Changing the heaviness (mass per meter) of the string. Heavier strings have greater mass per meter, and wave speed is slower. Since the wavelength remains constant heavier strings produce lower frequency (or the converse is true). 2. Increase the tension of the string. Increased tension increases the speed of the wave. Since the wavelength remains constant the frequency increases (or the converse is true). 1 Correct factors 1 Either factor and the corresponding explanation is correct. 1 Both factors and the corresponding explanations are correct.

A first order bright fringe is seen at a point on a screen. Constructive State the type of interference Explain, in terms of path difference, how the first order bright fringe is formed. Diffracted waves from the grating meet at the point P in phase. Since P is the first order bright fringe the waves meeting at this point have path difference of one wavelength. And that was a type 1 merit !!!!!!!!!!!!!!!!!!

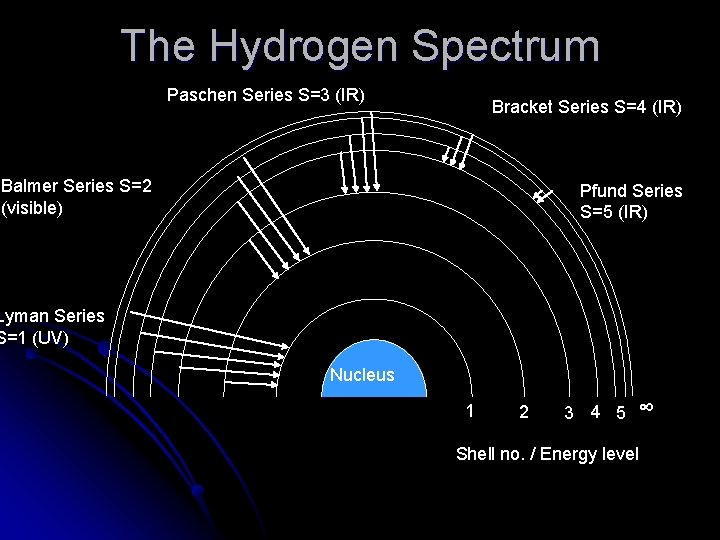
The Hydrogen Spectrum Balmer studied the emission spectrum lines of Hydrogen, as it is the simplest atom. l He was limited by the fact that he could only observe visible frequencies – we now know there are UV and IR spectral lines l

The Hydrogen Spectrum In Balmer’s case he was looking at spectral lines caused by electrons jumping from higher energy level (shells) down into the 2 nd shell. l They would release their extra energy as a photon of light. l Other Scientists later found series of spectral lines corresponding to jumps into the 1 st, 3 rd, 4 th, 5 th etc l

Quick verbal recap Concentrate and answer these Making an educated guess is better than “ I dunno” õ So what is an atomic absorption spectra? õAnd an emission spectra? õThey are useful because……………… õWhich spectra were discovered first? õBecause……………………… õ What level do the elctrons end up at for the visible spectra? õWhat is this series called?

The Hydrogen Spectrum Paschen Series S=3 (IR) Bracket Series S=4 (IR) Balmer Series S=2 (visible) Pfund Series S=5 (IR) Lyman Series S=1 (UV) Nucleus 1 2 3 4 5 ∞ Shell no. / Energy level

The Hydrogen Spectrum l A formula was worked out to calculate the wavelengths of these lines: R=Rydberg’s Constant=1. 097 x 10 -7 l S=Series no. (the shell jumped into) l L=Line no. (the shell jumped from) l

The Hydrogen Spectrum The formula worked perfectly for Hydrogen, but started to get more inaccurate the bigger and more complex the atom got l Absorption spectra are produced by electrons absorbing photons of energy which allows them to jump up energy levels l

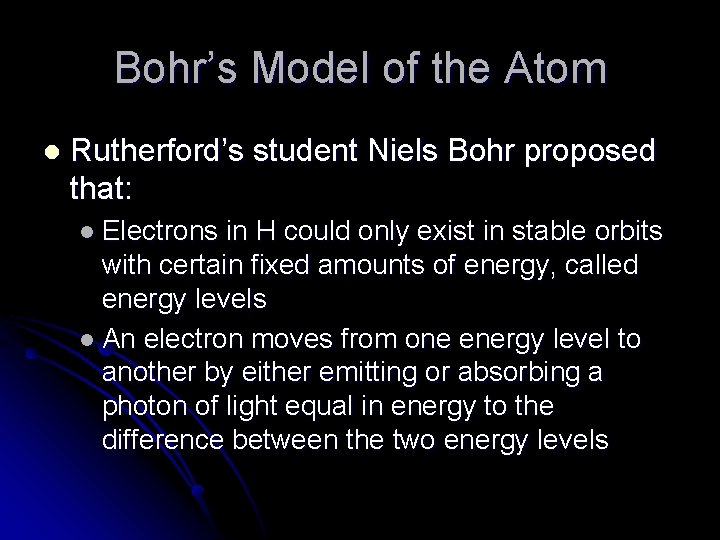
Bohr’s Model of the Atom l Rutherford’s student Niels Bohr proposed that: l Electrons in H could only exist in stable orbits with certain fixed amounts of energy, called energy levels l An electron moves from one energy level to another by either emitting or absorbing a photon of light equal in energy to the difference between the two energy levels

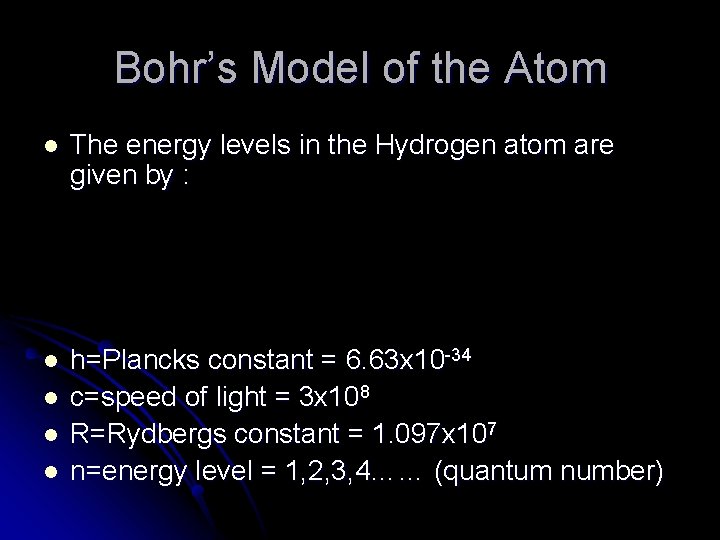
Bohr’s Model of the Atom l The energy levels in the Hydrogen atom are given by : l h=Plancks constant = 6. 63 x 10 -34 c=speed of light = 3 x 108 R=Rydbergs constant = 1. 097 x 107 n=energy level = 1, 2, 3, 4…… (quantum number) l l l

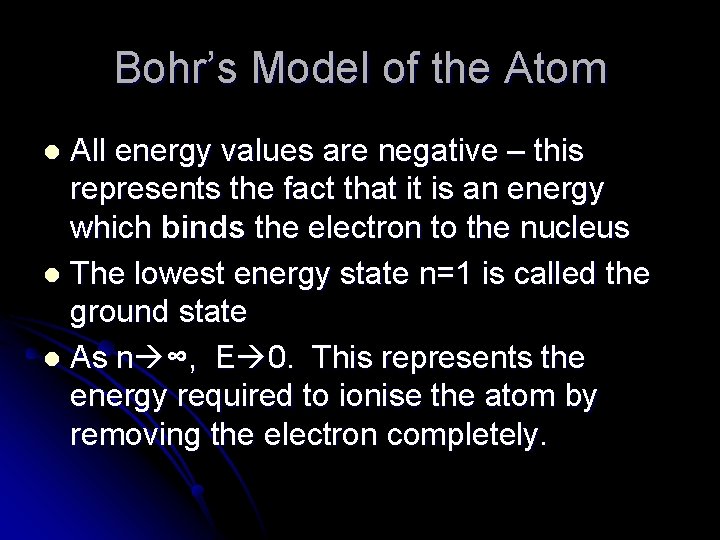
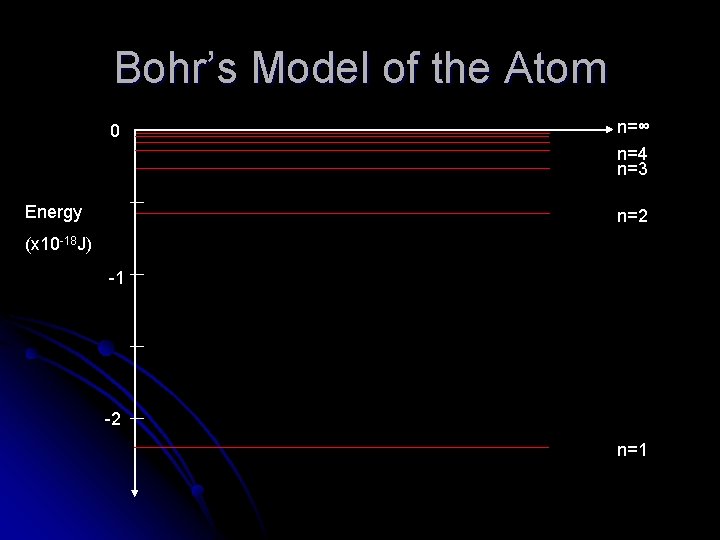
Bohr’s Model of the Atom All energy values are negative – this represents the fact that it is an energy which binds the electron to the nucleus l The lowest energy state n=1 is called the ground state l As n ∞, E 0. This represents the energy required to ionise the atom by removing the electron completely. l

Recap What energy level transition does an electron make to produce a visible emission? Higher level 2 Why do electron transitions It’s the largest energy to level 1 produce UV? jump and UV in a larger amount of energy than visible or IR What were Bohrs 2 assumptions about the H atom Nip back 3 slides Use Bohrs Energy level formula to work the energy of the first level.

Bohr’s Model of the Atom 0 Energy n=∞ n=4 n=3 n=2 (x 10 -18 J) -1 -2 n=1

Electron Volts Sometimes an alternative unit for energy is used called the electron volt l 1 e. V is the energy gained by 1 electron when accelerated by a potential of 1 Volt l 1 e. V=1. 6 x 10 -19 J l Using this unit: l

Balancing Nuclear Equations l l The atomic numbers are conserved. That means they have to add up to the same number on both sides of the equation. The same applies to the atomic masses. ? Note: Both mass and charge must be conserved

Try this (ie 226=222+4, 88=86+2)

Another One l Cobalt 60 decays to Nickel 60 emitting a a gamma ray and ? l. Again, mass and charge are conserved l. NB. the a or b symbols can be used instead of He or e Why have 2 different atoms got the same atomic mass? (type 1 question)

Last one for now That’s how the nucleus is able to emit electrons What is bigger a proton or neutron? (Type 1 question again) Think about it and don’t yell out

Binding Energy If we put together a nucleus from individual protons and neutrons, we would find that the mass of the resulting nucleus is less than the total mass of the individual nucleons. l This reduction in mass is called a mass deficit l

Binding Energy In order to break up a nucleus into separate nucleons the mass deficit must be restored by adding extra energy. l This energy changes into mass according to Einstein’s famous equation: l

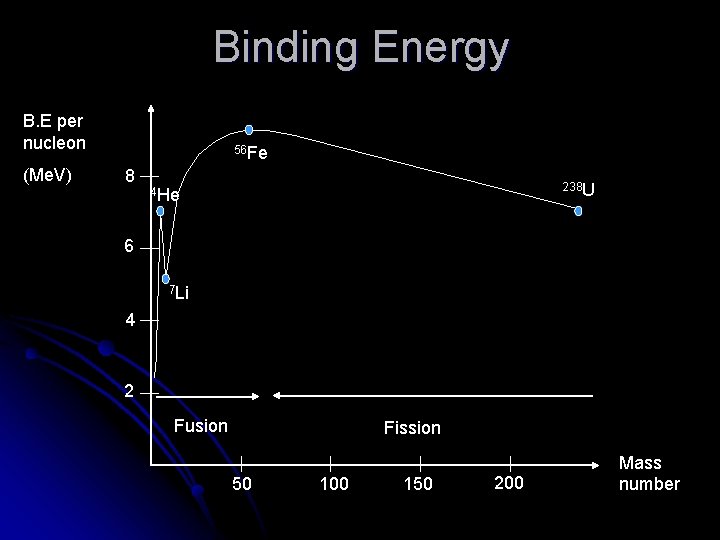
Binding Energy This energy shortage has the effect of holding the nucleus together so it is called the binding energy. l Binding energy represents the amount of “glue” holding the nucleus together. l The more binding energy per nucleon, the more stable an atom will be l

Binding Energy B. E per nucleon (Me. V) 56 Fe 8 238 U 4 He 6 7 Li 4 2 Fusion Fission 50 100 150 200 Mass number

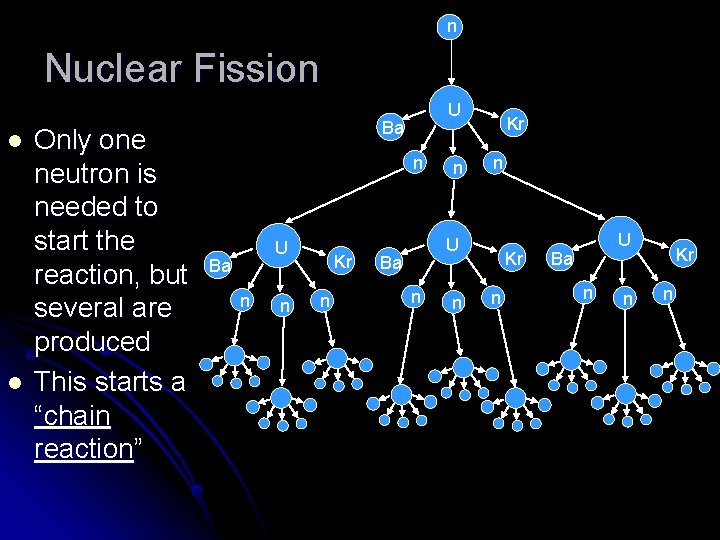
Nuclear Fission Breaking large unstable nuclei into smaller ones. l Lots of possible combinations of fragments from one initial nucleus l Eg: l ?

Nuclear Fission When a large nucleus is split into smaller fragments, the fragments have less mass per nucleon (see graph p 377 text) l The lost mass is released as energy in the form of kinetic energy of neutrons and gamma rays l

n Nuclear Fission l l Only one neutron is needed to start the reaction, but several are produced This starts a “chain reaction” U Ba n n Kr n

Nuclear Fission If the chain reaction is controlled it can be used in a nuclear reactor l If it is uncontrolled it explodes as a nuclear or atomic bomb l

Nuclear Fusion The joining of two small nuclei to form one larger one l This is the process that powers the sun l Eg: l

Nuclear Fusion Fusing two light atoms together results in a nucleus with less mass per nucleon l The lost mass results in a release of energy l

Nuclear Fusion requires extreme temperature (eg millions of degrees) to occur, and has not practically and economically been used in power generation (yet…. ) l Hydrogen bombs have been successfully made, but require a fission reaction to provide the necessary temp. l So that’s it folks Here endth the lesson as they say Just need to consolidate And revise for the 20 th

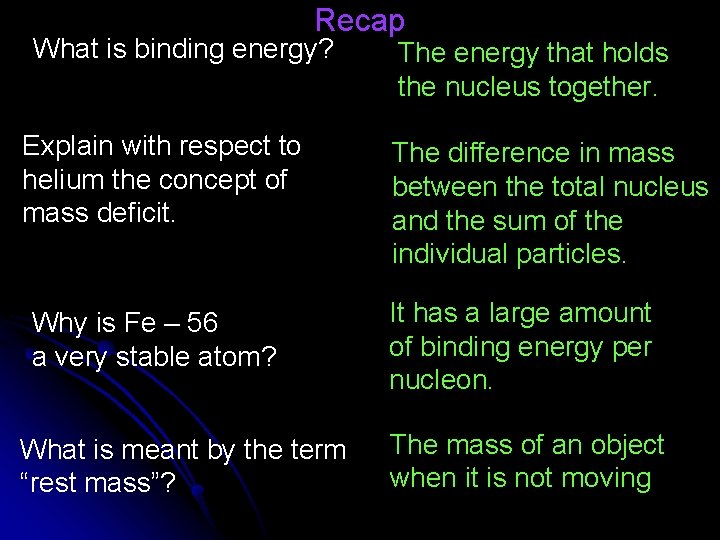
Recap What is binding energy? Explain with respect to helium the concept of mass deficit. Why is Fe – 56 a very stable atom? What is meant by the term “rest mass”? The energy that holds the nucleus together. The difference in mass between the total nucleus and the sum of the individual particles. It has a large amount of binding energy per nucleon. The mass of an object when it is not moving

Rest Masses: Alpha particle 6. 64591 x 10 -27 kg Neutron 1. 67483 x 10 -27 kg proton 1. 67338 x 10 -27 kg What is the rest mass of 2 protons and 2 neutrons? 6. 69642 x 10 -27 kg So what is the binding energy for an alpha particle? Work out the change in mass then use 2 to get E=mc The first nuclear bomb killed about 250000 people by releasing the nuclear binding energy of 1 g of mass. How much energy was released? 4. 5459 x 10 -12 J Using E=mc 2 9 x 1013 J

Nuclear Physics Revision What are the 2 forces acting between protons? Nuclear and electric Which one is larger? What is the difference between Random/natural spontaneous radio activity and fission? Man-made A H atom is in its ground state What does that mean? Electrons are in The n=1 energy level

Problem- Serious Challenge Blue light wavelength 429 nm is shone on a metal surface with threshold frequency 3. 0 x 1014 Hz. What is the maximum velocity of the electrons (mass=9. 1 x 10 -31 kg)? Step one find the freq. of the light. 7. 0 x 1014 Hz Step two calc the photon energy 4. 6 x 10 -19 J Use hfo=Φ, Ek=hf- Φ, Ek=½mv 2 to get v v=7. 6 x 105 ms-1

The electron of a H atom makes the transition from E 4 to E 3. Is a photon emitted or absorbed? Emitted. When the electron makes the transition from E 4 to E 2 is a higher or lower frequency photon emitted than above? Higher. How much energy is required to ionise an electron in E 4 of a H atom? Using E=-Rch/n 2 1. 36 x 10 -19 J If an electron is in E 4 how many different frequencies of light can be emitted as the atom loses its energy? 3. What wavelength of light is emitted from an electron moving from E 3 E 2? Using Rydbergs formula 656 nm

You need to know the details about each series, so……Zeldis – who named the visible series? Balmer-great… Q-in what level do the electrons fall to in this series? 2 -cool… Robert what level do the electrons fall to to produce infra-red light? 3, 4, 5 -smokin… James what is the energy level electrons end up at with the Lyman Series? 1 -outstanding… Jane which series has higher energy photons Paschen or Bracket? Paschen-stunning… Joanne what must a L number start at? S+1 - correct-as expected

If you are working out the third line in the Lyman series emission spectrum what numbers would you use for L and S? L=4 and S=1 What sort of emission would this be? UV What is the S. I. unit for energy? Joule How many neutrons are there in tritium 2 and deuterium? 1

Just Read This Question Three The Hydrogen Atom While at the Conference, Makalesi visited the Advanced Physics Laboratory of the University hosting the Conference. She observed the atomic line spectra of a hydrogen atom using a spectrometer to view light from a hydrogen-filled discharge tube. The third year student who demonstrated this used the terms ionisation and excitation energy in his talk to explain the spectra. Qu 3 2004 NZIP

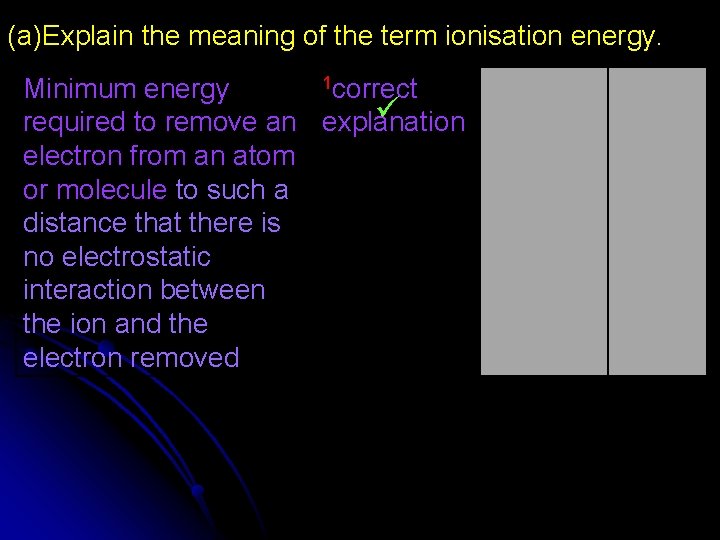
(a)Explain the meaning of the term ionisation energy. 1 correct Minimum energy required to remove an explanation electron from an atom or molecule to such a distance that there is no electrostatic interaction between the ion and the electron removed

(b) What is the difference between ionisation and excitation energy? 1 correct Ionisation energy removes electron answer from atom, excitation shifts electron to higher energy levels within the atom

Makalesi saw visible light at one point in the experiment. (c) Explain, in terms of electron movement, how visible light would be produced in this experiment. This is produced when an excited electron jumps from the 3 rd or higher excited energy level and drops down to the second excited energy level emitting photons of visible light. 1 Replacement 1 correct answer

(d) Name two other parts of the electromagnetic spectrum that can be emitted in this experiment. UV and infrared correct answer 1 (e)Calculate the frequency of the radiation when an electron in the hydrogen atom jumps from third to first excited energy level. Use Rydberg’s constant as 1. 097 x 107 m-1. (f)

Get into the habit of getting the correct answers