Modern Periodic Table The Periodic Law In the
Modern Periodic Table
The Periodic Law • In the modern periodic table, elements are arranged by increasing atomic number (increasing number of protons)
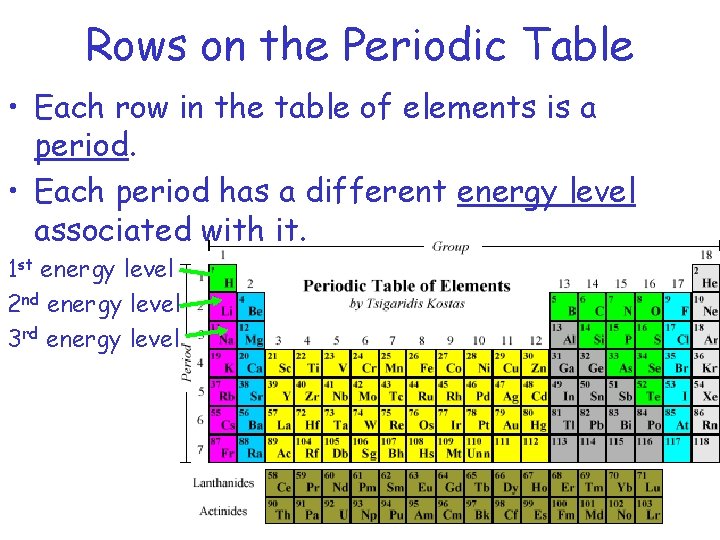
Rows on the Periodic Table • Each row in the table of elements is a period. • Each period has a different energy level associated with it. 1 st energy level 2 nd energy level 3 rd energy level

Columns on the Periodic Table • Each column on the periodic table is called a group or family. • Properties of elements repeat in a predictable way when atomic numbers are used to arrange elements into groups.
Periodic Law • Elements in a group have similar electron configurations. • Elements in a group have similar chemical properties. • The pattern of repeating properties is called periodic law.
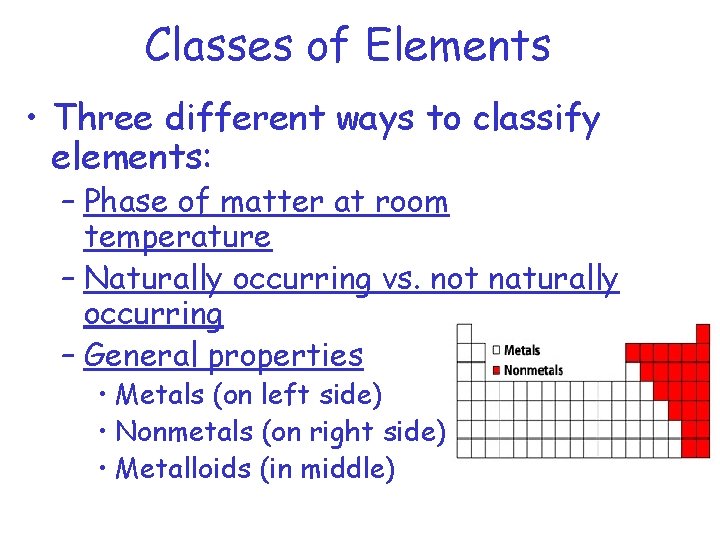
Classes of Elements • Three different ways to classify elements: – Phase of matter at room temperature – Naturally occurring vs. not naturally occurring – General properties • Metals (on left side) • Nonmetals (on right side) • Metalloids (in middle)

Metals • • • Elements that are good conductors. Solid at room temperature (except Mercury) Most are malleable. Many are ductile (drawn into wires) Transition metals (groups 3 -12) – Form a bridge between the left and right sides of table. – Form compounds with distinctive colors. – cool reactions – more cool reactions

Nonmetals • Elements that are poor conductors • Elements have low boiling points • Many are gases at room temperature • Some are solids at room temperature but are very brittle.

Metalloids • Properties of this group vary. • For example, a metalloids ability to conduct electricity varies with temperature.

Variation Across a Period • Across a period, from left to right, the elements become less metallic and more nonmetallic in their properties. • Most reactive metals on left side • Most reactive non-metals on the right side • more cool reactions
- Slides: 11