Modern Periodic Table Chapter 5 Dimitri Mendeleev Developed
Modern Periodic Table Chapter 5
Dimitri Mendeleev • Developed the modern periodic system • Placed elements in order of increasing atomic number • Reversed the order of some elements to keep similar elements in the same column • Ex. Ar and K • Predicted the existence and properties of elements that had not yet been discovered-this demonstrated the value of the table
Modern Periodic Table • Elements are placed in order of increasing atomic number • Periodic Law - the phys. and chem. properties of the elements are periodic functions of their atomic numbers.

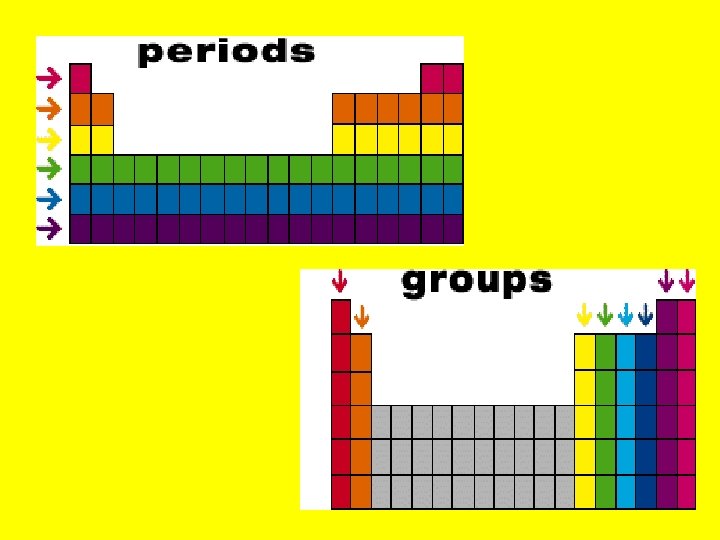
Overview of Periodic Table • Horizontal rows are called periods • The period number for an element will tell you where its outermost electrons are located. • Ex. • Li -1 s 22 s 1 • B- 1 s 22 p 1 **the 2 nd energy level is the outermost for both • Vertical columns are called groups (elements in a group have similar properties) • Ex. Group 1 elements are all highly reactive
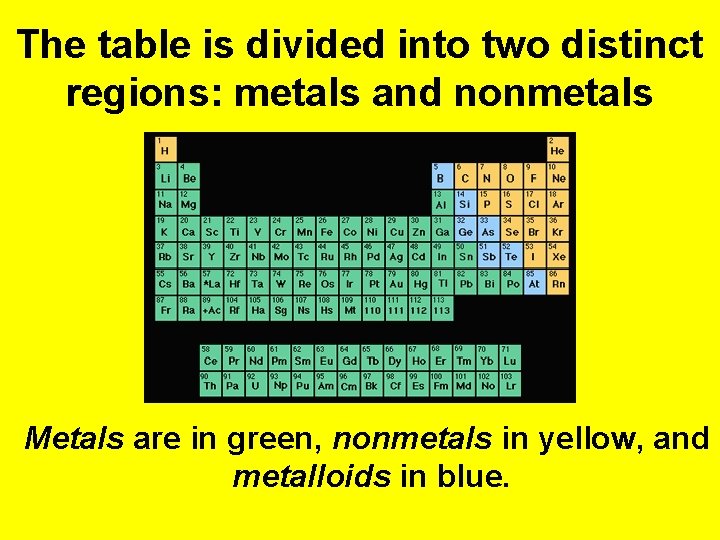
The table is divided into two distinct regions: metals and nonmetals Metals are in green, nonmetals in yellow, and metalloids in blue.

Properties of Metals • Good conductors of heat and electricity • Most are solids at room temp. - the only exception is Hg (mercury!) • Malleable-can be bent • Ductile- can be drawn into a wire

Properties of Nonmetals • Poor conductors of heat and electricity • Not malleable or ductile • Most are gases or liquids at room temp.
Properties of Metalloids • Have properties of both metals and nonmetals (can conduct heat and electricity, just not very well!) • Also called semiconductors

Main Group Elements • Groups 1, 2, and 13 -18 • Sometimes called the representative elements
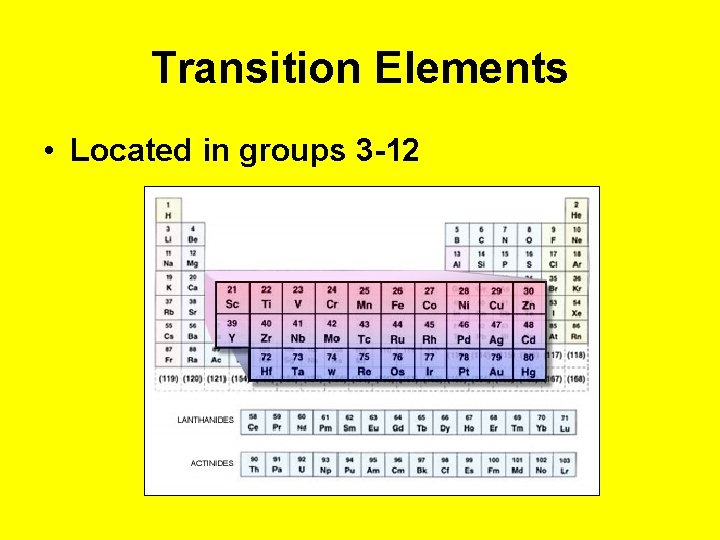
Transition Elements • Located in groups 3 -12

Alkali Metals • React w/water to produce alkaline solutions and have metallic properties • Soft • Have shiny surfaces • Highly reactive • Become plasmas in gaseous state • All located in Group 1 Sodium, Na Potassium, K

Alkaline Earth Metals • Harder, denser, and stronger than alkali metals • Less reactive than alkali metals • All located in Group 2 Calcium, Ca Magnesium, Mg

Halogens • Halogen is derived from a Greek word that means “salt former” • These elements combine w/metals to form salts (ex: salt Na. Cl) • Most reactive group of nonmetals • All located in Group 17 Chlorine Bromine

Noble Gases • Formerly known as inert gases because they were believed to be completely unreactive • Are unreactive in nature because of their full outermost energy levels • All located in Group 18
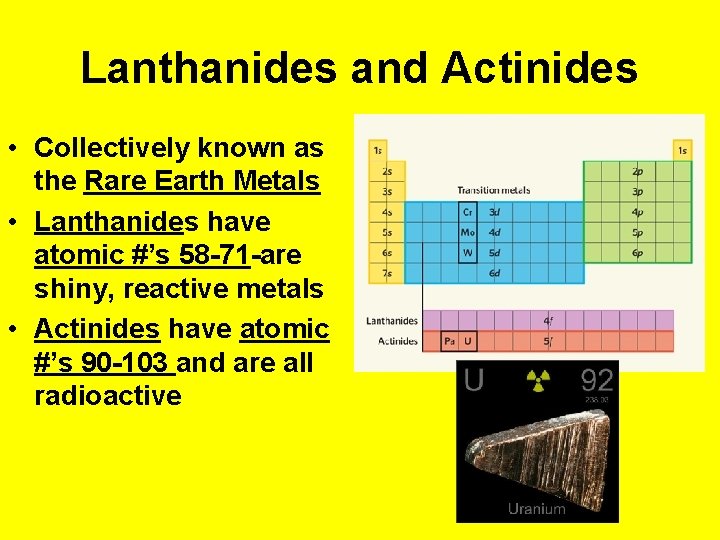
Lanthanides and Actinides • Collectively known as the Rare Earth Metals • Lanthanides have atomic #’s 58 -71 -are shiny, reactive metals • Actinides have atomic #’s 90 -103 and are all radioactive

Hydrogen • The most common element in the universe • Unique behavior due to the fact that it only has 1 proton and 1 electron • Can react w/many elements


Many properties of elements change in predictable ways: • There are six periodic trends we will talk about • • • Atomic Radii Ionization Energy Electron Affinity Electronegativity Valence Electrons
Atomic Radius • “The distance from the center of an atom’s nucleus to its outermost electron” • Atoms get larger down a group • Atoms get smaller across a period • Why? • As you move down a group, there are more principle energy levels. • As you move across a period, the attraction between the increasing # of protons and electrons causes the atom to shrink.

Which has a larger atomic radii? Oxygen or Nitrogen? Oxygen has a smaller radii because there are more electrons and protons, hence a stronger attraction, which pulls the atom closer together • Nitrogen Atom: • Oxygen Atom:
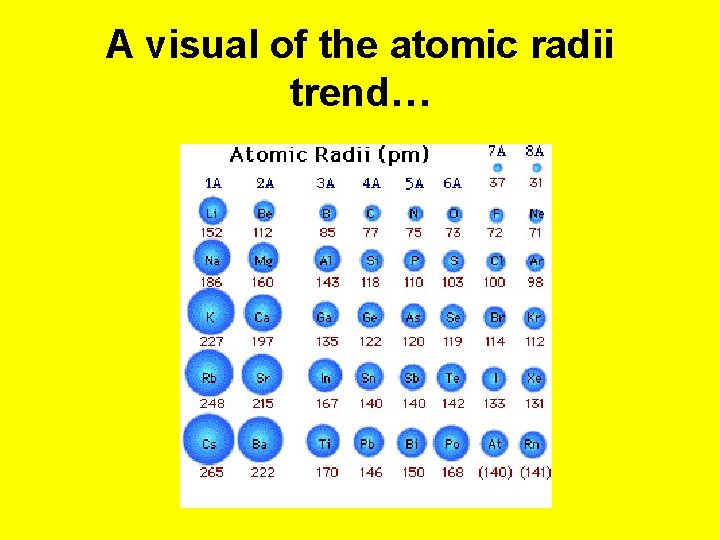
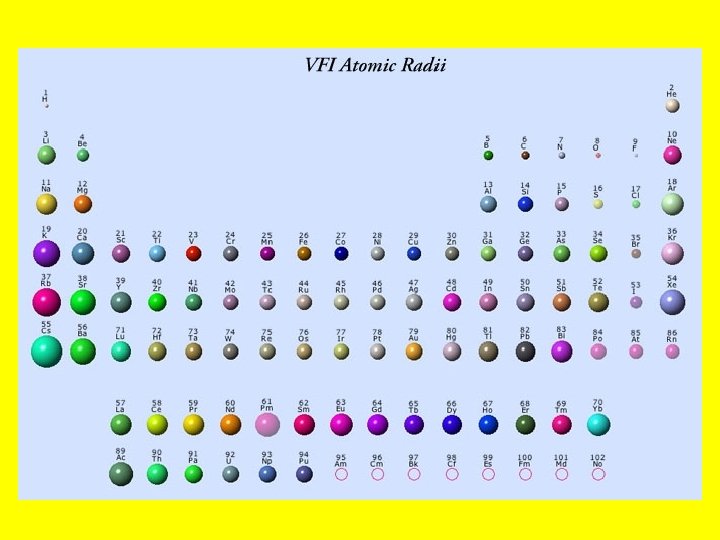
A visual of the atomic radii trend…
Atomic Radius Examples • 1. 2. 3. 4. Who has the larger radius: Li or Rb Ca or Br Be or Ba Al or Ga Answers: 1. Rb 2. Ca 3. Ba 4. Ga
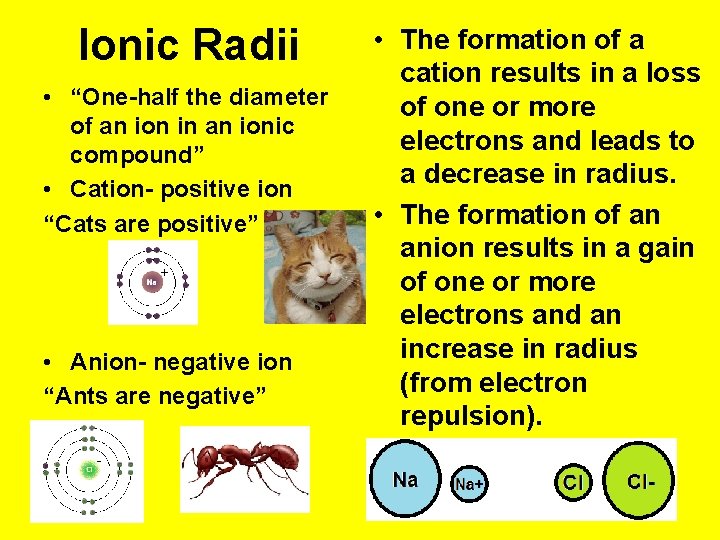
Ionic Radii • “One-half the diameter of an ion in an ionic compound” • Cation- positive ion “Cats are positive” • Anion- negative ion “Ants are negative” • The formation of a cation results in a loss of one or more electrons and leads to a decrease in radius. • The formation of an anion results in a gain of one or more electrons and an increase in radius (from electron repulsion).
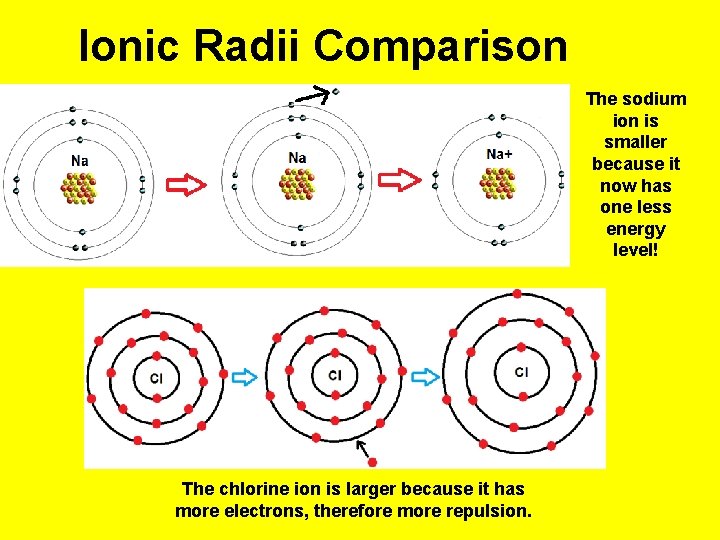
Ionic Radii Comparison The sodium ion is smaller because it now has one less energy level! The chlorine ion is larger because it has more electrons, therefore more repulsion.
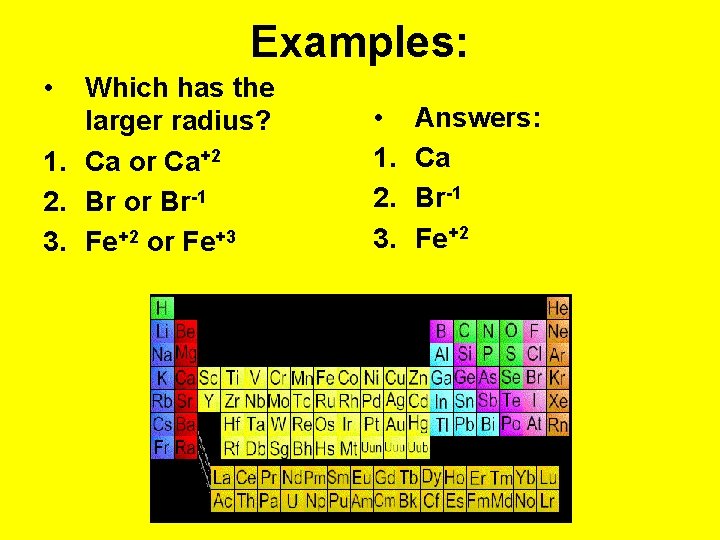
Examples: • Which has the larger radius? 1. Ca or Ca+2 2. Br or Br-1 3. Fe+2 or Fe+3 • 1. 2. 3. Answers: Ca Br-1 Fe+2
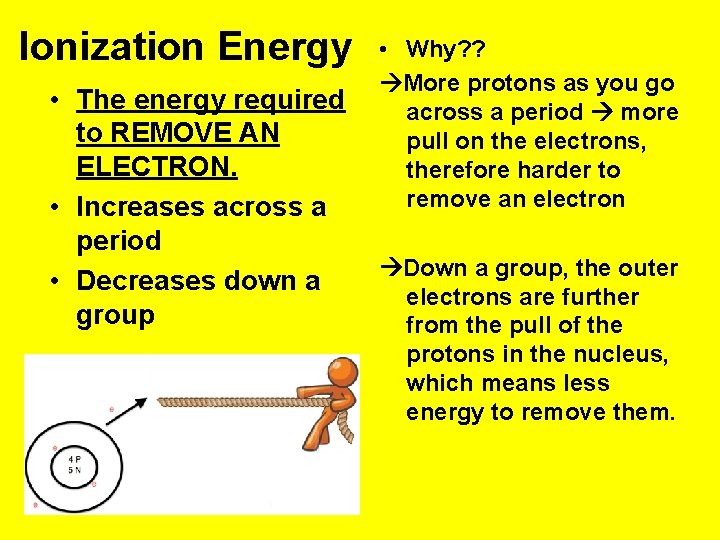
Ionization Energy • The energy required to REMOVE AN ELECTRON. • Increases across a period • Decreases down a group • Why? ? More protons as you go across a period more pull on the electrons, therefore harder to remove an electron Down a group, the outer electrons are further from the pull of the protons in the nucleus, which means less energy to remove them.
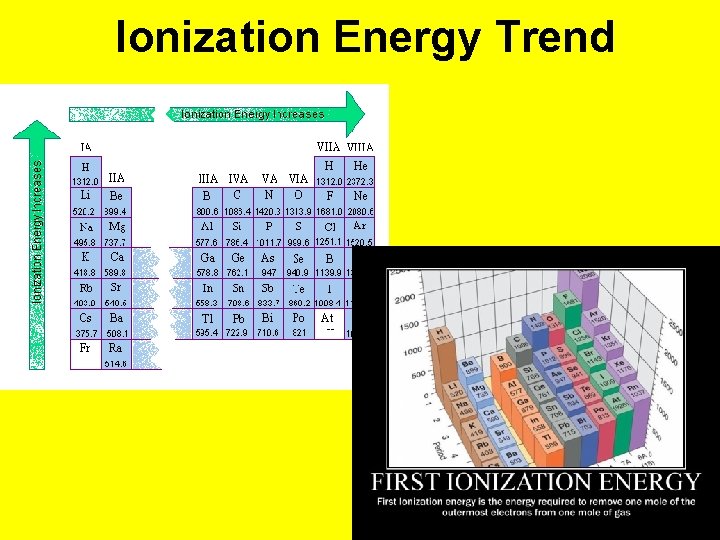
Ionization Energy Trend
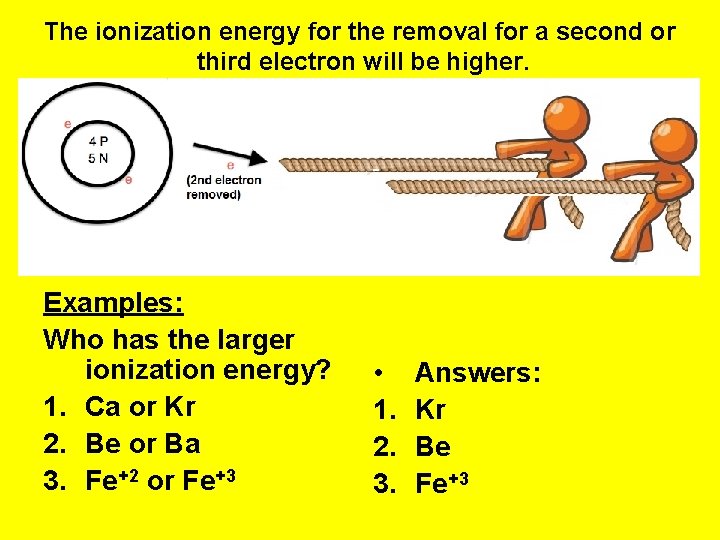
The ionization energy for the removal for a second or third electron will be higher. Examples: Who has the larger ionization energy? 1. Ca or Kr 2. Be or Ba 3. Fe+2 or Fe+3 • 1. 2. 3. Answers: Kr Be Fe+3
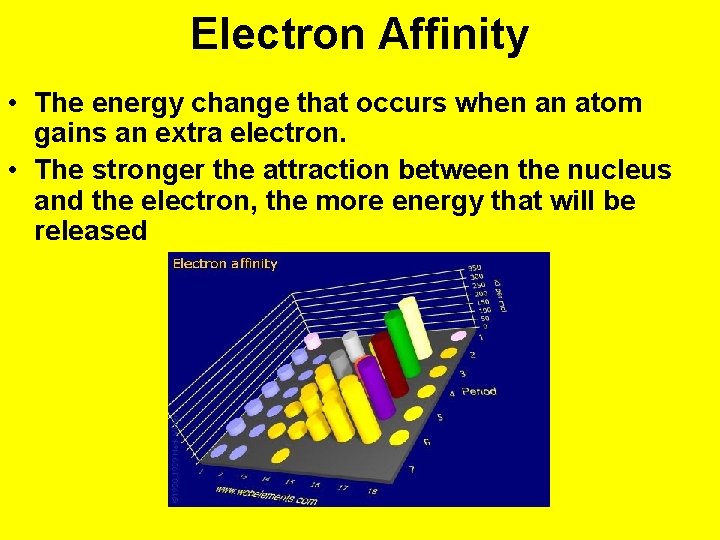
Electron Affinity • The energy change that occurs when an atom gains an extra electron. • The stronger the attraction between the nucleus and the electron, the more energy that will be released
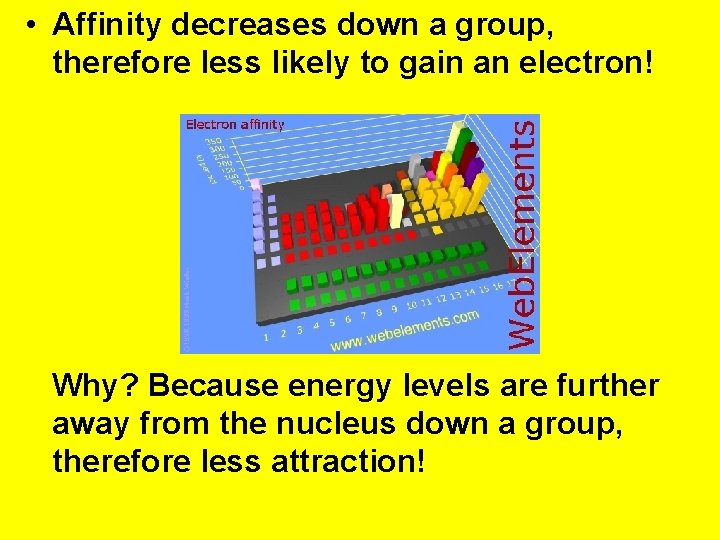
• Affinity decreases down a group, therefore less likely to gain an electron! Why? Because energy levels are further away from the nucleus down a group, therefore less attraction!
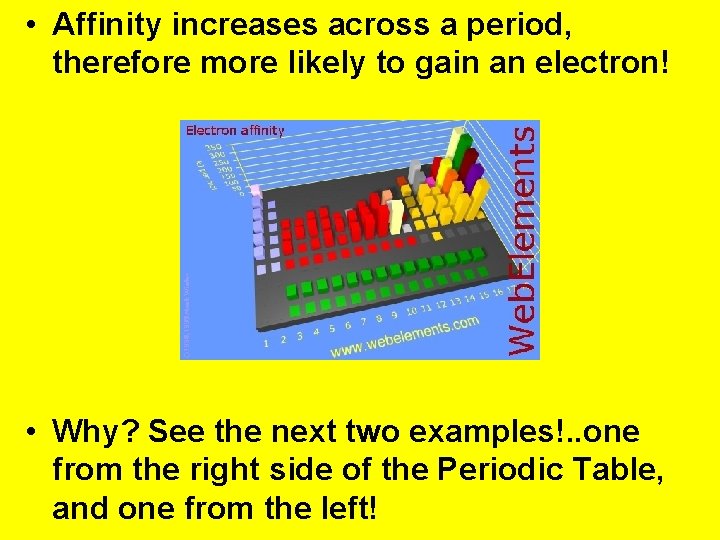
• Affinity increases across a period, therefore more likely to gain an electron! • Why? See the next two examples!. . one from the right side of the Periodic Table, and one from the left!
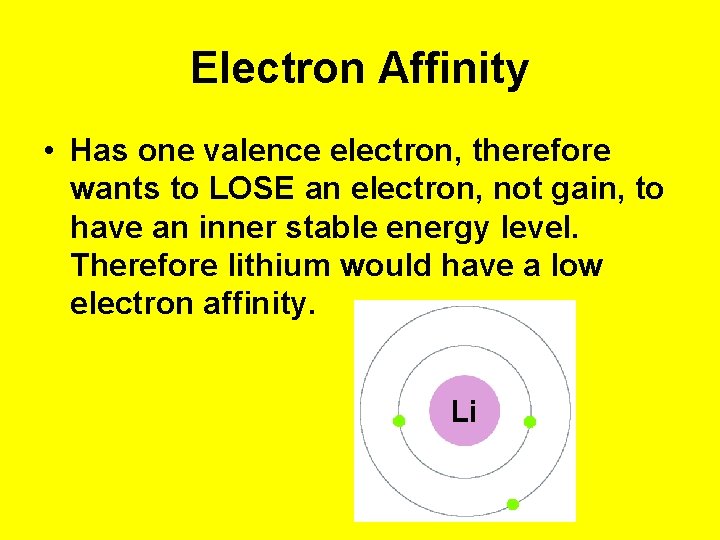
Electron Affinity • Has one valence electron, therefore wants to LOSE an electron, not gain, to have an inner stable energy level. Therefore lithium would have a low electron affinity.

Electron Affinity • Has seven valence electrons, therefore wants to GAIN an electron, not lose, to have an outer stable energy level. Therefore fluorine would have a high electron affinity.

Electron Affinity Examples: • Which has a higher electron affinity? 1. Mg or Cl 2. F or At Answers: 1. Cl 2. F
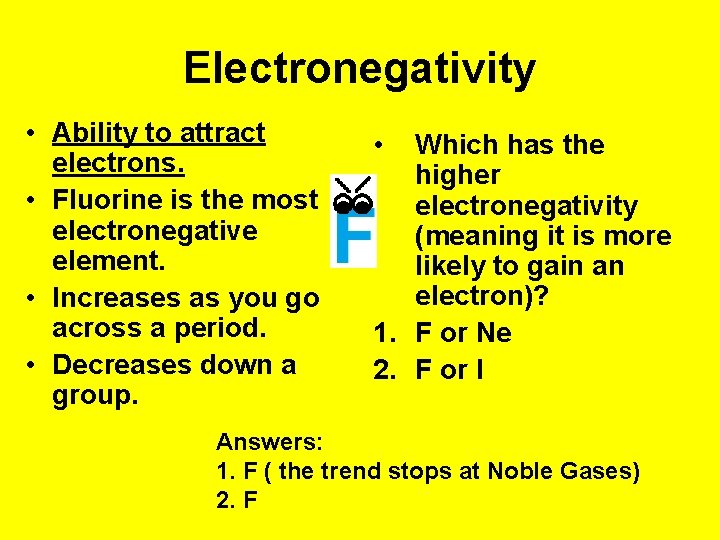
Electronegativity • Ability to attract electrons. • Fluorine is the most electronegative element. • Increases as you go across a period. • Decreases down a group. • Which has the higher electronegativity (meaning it is more likely to gain an electron)? 1. F or Ne 2. F or I Answers: 1. F ( the trend stops at Noble Gases) 2. F
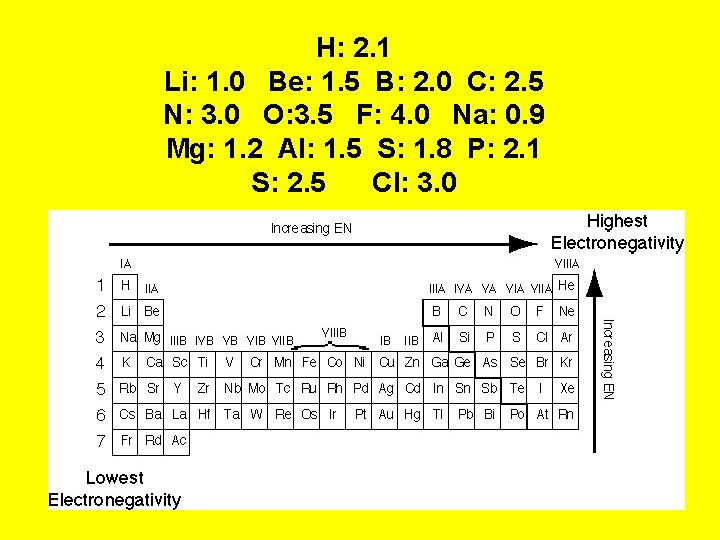
H: 2. 1 Li: 1. 0 Be: 1. 5 B: 2. 0 C: 2. 5 N: 3. 0 O: 3. 5 F: 4. 0 Na: 0. 9 Mg: 1. 2 Al: 1. 5 S: 1. 8 P: 2. 1 S: 2. 5 Cl: 3. 0
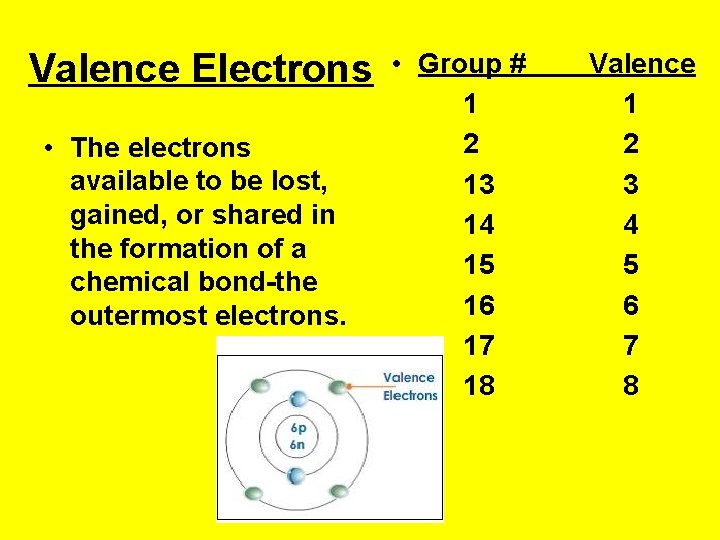
Valence Electrons • The electrons available to be lost, gained, or shared in the formation of a chemical bond-the outermost electrons. • Group # 1 2 13 14 15 16 17 18 Valence 1 2 3 4 5 6 7 8
- Slides: 39