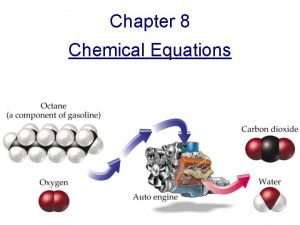
Modern Chemistry Chapter 8 Chemical Equations and Reactions









































- Slides: 71

Modern Chemistry Chapter 8 Chemical Equations and Reactions 1

Section 1 Describing Chemical Reactions 2

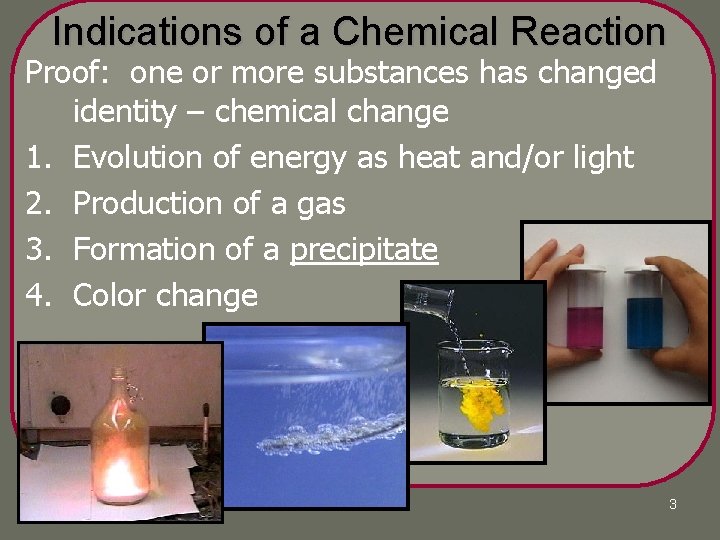
Indications of a Chemical Reaction Proof: one or more substances has changed identity – chemical change 1. Evolution of energy as heat and/or light 2. Production of a gas 3. Formation of a precipitate 4. Color change 3

4 Indications of a Reaction

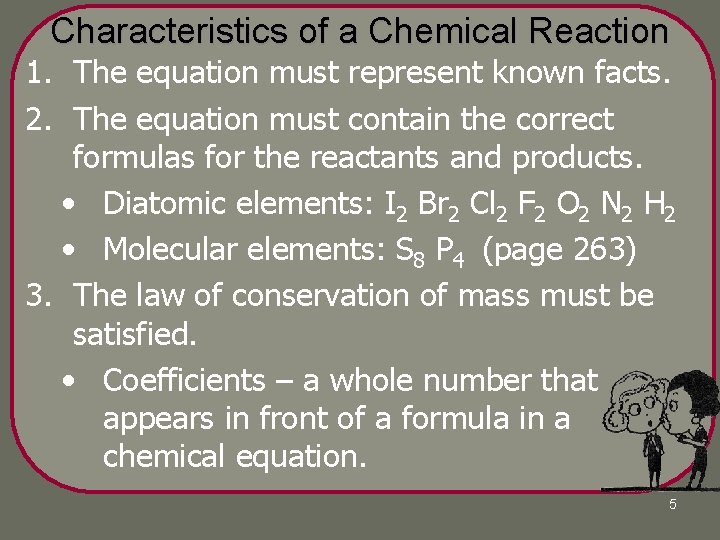
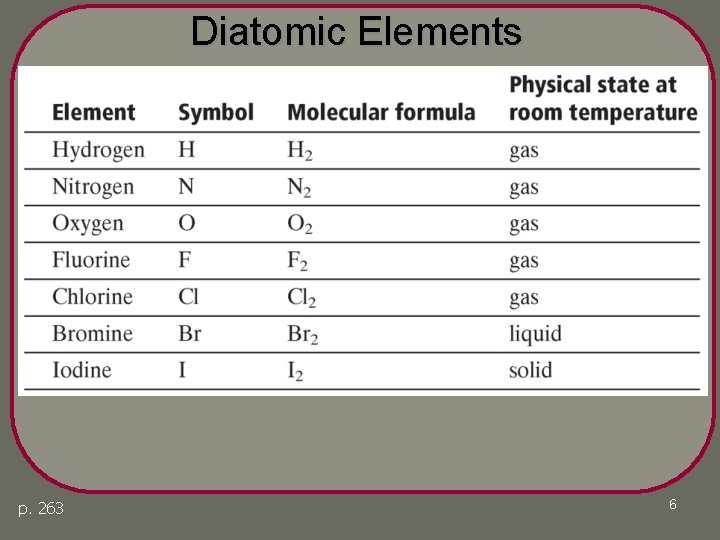
Characteristics of a Chemical Reaction 1. The equation must represent known facts. 2. The equation must contain the correct formulas for the reactants and products. • Diatomic elements: I 2 Br 2 Cl 2 F 2 O 2 N 2 H 2 • Molecular elements: S 8 P 4 (page 263) 3. The law of conservation of mass must be satisfied. • Coefficients – a whole number that appears in front of a formula in a chemical equation. 5

Diatomic Elements p. 263 6

Nitrogen gas molecules

A Collection of Argon Atoms

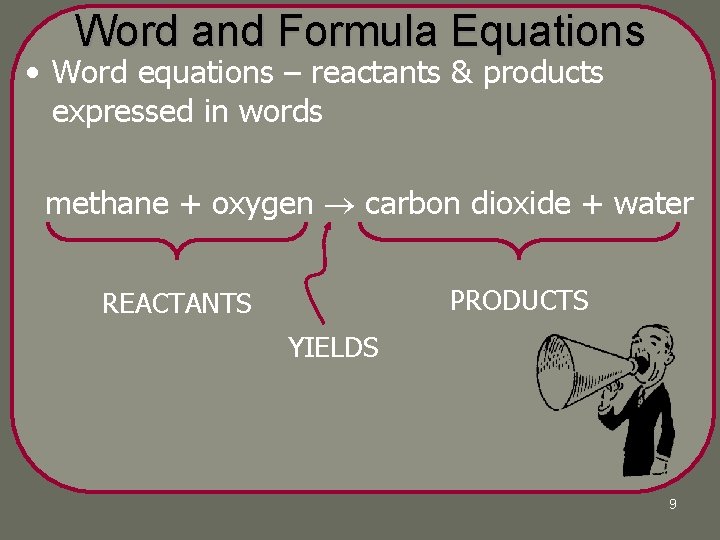
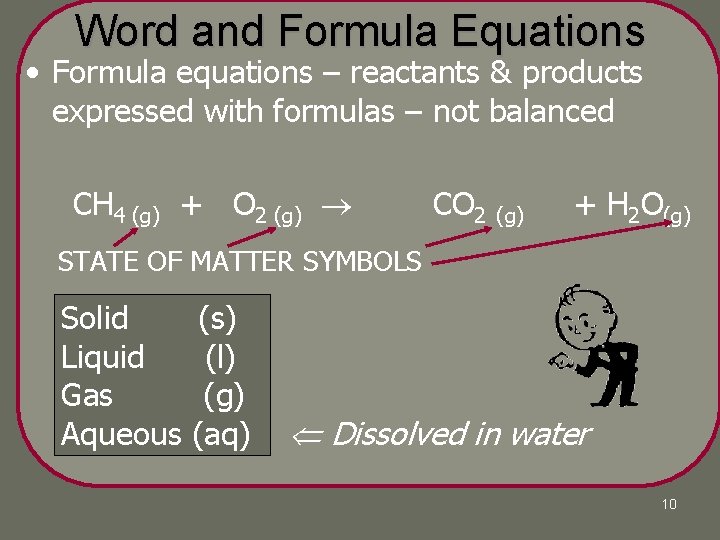
Word and Formula Equations • Word equations – reactants & products expressed in words methane + oxygen carbon dioxide + water PRODUCTS REACTANTS YIELDS 9

Word and Formula Equations • Formula equations – reactants & products expressed with formulas – not balanced methane + Hwater CH 4 (g) + oxygen O 2 (g) carbon COdioxide + 2 (g) 2 O(g) STATE OF MATTER SYMBOLS Solid Liquid Gas Aqueous (s) (l) (g) (aq) Dissolved in water 10

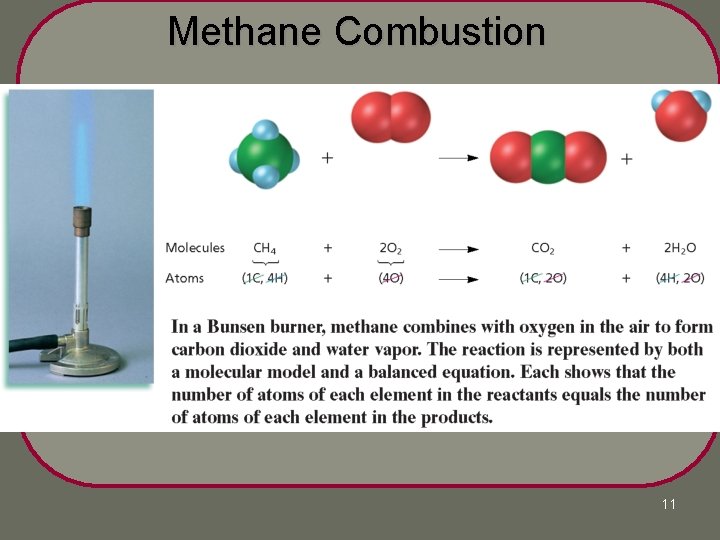
Methane Combustion 11

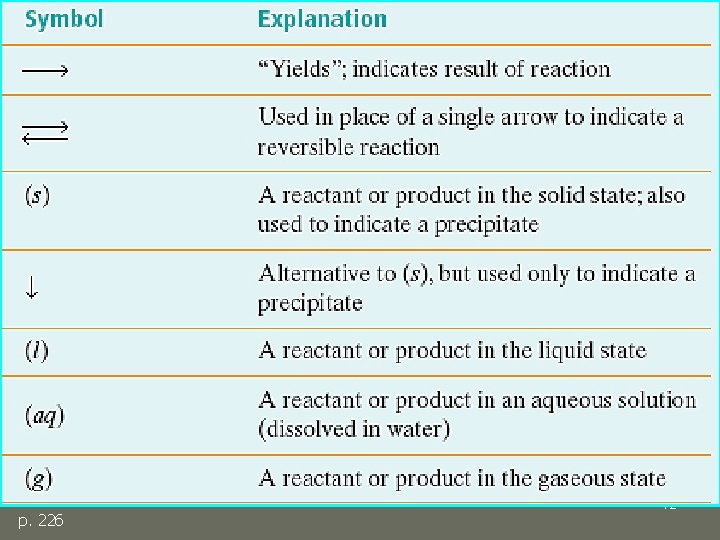
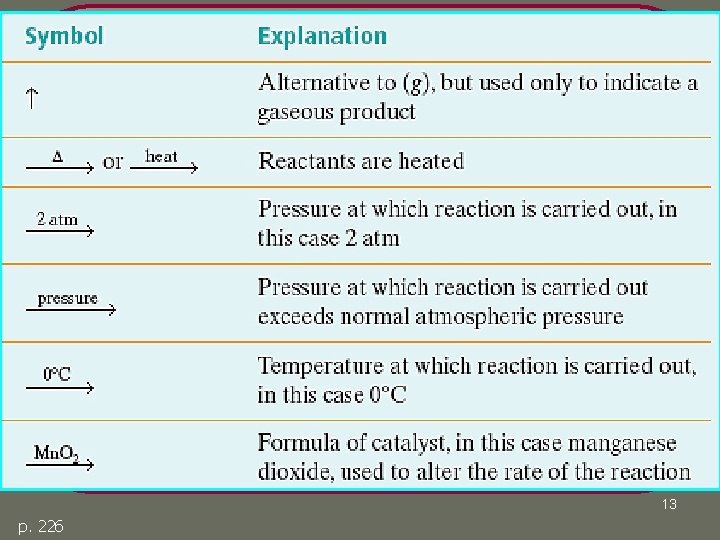
Additional Symbols p. 226 12

Additional Symbols 13 p. 226

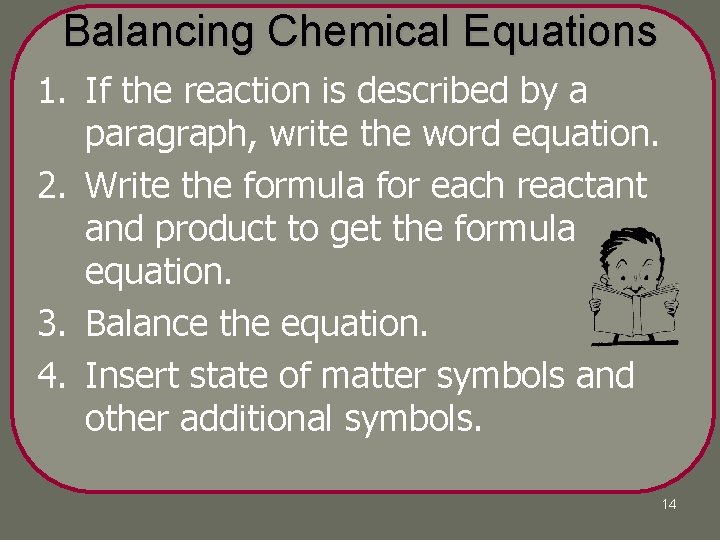
Balancing Chemical Equations 1. If the reaction is described by a paragraph, write the word equation. 2. Write the formula for each reactant and product to get the formula equation. 3. Balance the equation. 4. Insert state of matter symbols and other additional symbols. 14

Balancing Chemical Equations GOAL OF THE GAME: To get the same number of atom of each element in the reactant and the product. To obey the law of conservation of mass. 15

Balancing Chemical Equations RULES OF THE GAME: Only coefficients can be added or changed. Once formulas are written subscripts can not be changed. 16

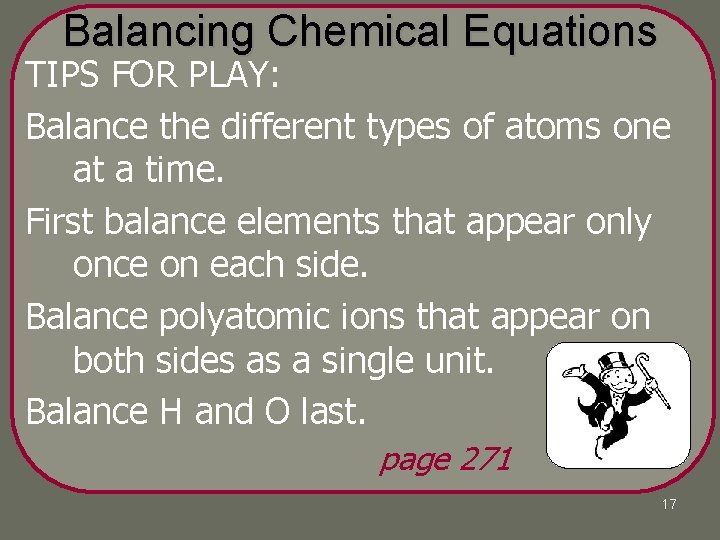
Balancing Chemical Equations TIPS FOR PLAY: Balance the different types of atoms one at a time. First balance elements that appear only once on each side. Balance polyatomic ions that appear on both sides as a single unit. Balance H and O last. page 271 17

Balancing Chemical Equations TIPS FOR PLAY: Try keeping a tally for each element on each side below the equation. If it could be balanced by a coefficient of 1½ - use it- multiply all coefficients in the equation by 2. 18

Sample Problem The reaction of zinc with aqueous hydrochloric acid produces a solution of zinc chloride and hydrogen gas. Write a balanced equation for the reaction 19

Sample Problem p. 272 zinc + hydrochloric acid zinc chloride + hydrogen Zn + HH Cl Cl Zn Zn 2 HCl Zn. Cl 2 (s) + 2 HCl (aq) Cl Zn Cl + H 2 H H Zn. Cl 2 (aq) + H 2 (g) 20

Writing Equations Two atoms of aluminum react with three units of aqueous copper(II) chloride to produce three atoms of copper and two units of aqueous aluminum chloride. • How many? • Of what? • In what state?

Writing Equations 2 Al (s) + 3 Cu. Cl 2 (aq) 3 Cu (s) + 2 Al. Cl 3 (aq)

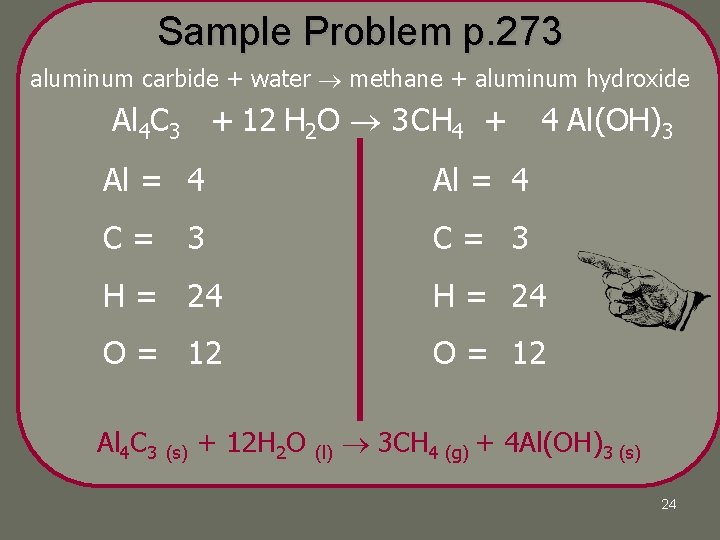
Sample Problem Solid aluminum carbide, reacts with water to produce methane gas, CH 4, and solid aluminum hydroxide. Write a balanced chemical equation for this reaction. 23

Sample Problem p. 273 aluminum carbide + water methane + aluminum hydroxide Al 4 C 3 + 12 H 2 O 3 CH 4 + 4 Al(OH)3 Al = 4 1 C= 3 1 H = 24 2 H = 24 7 16 O = 12 1 O = 12 3 Al 4 C 3 (s) + 12 H 2 O (l) 3 CH 4 (g) + 4 Al(OH)3 (s) 24

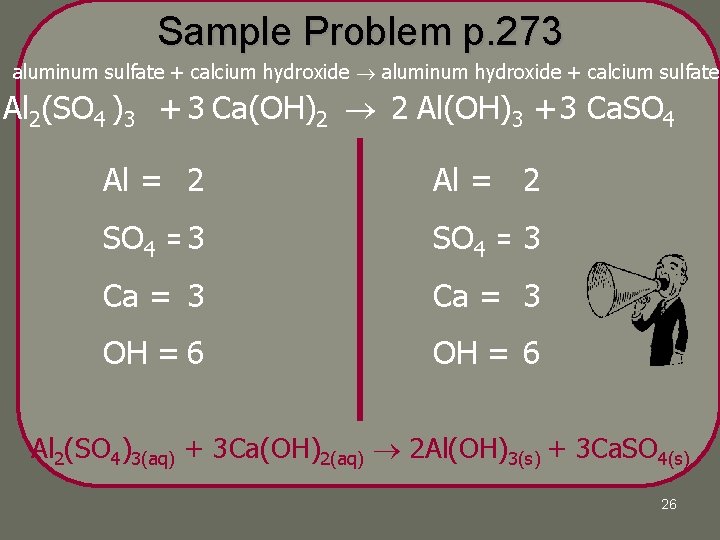
Sample Problem Aluminum sulfate and calcium hydroxide are used in water-purification process. When added to water, they dissolve and react to produce two insoluble products, aluminum hydroxide and calcium sulfate. These products settle out, taking suspended solid impurities with them. Write a balanced chemical equation for the reaction. 25

Sample Problem p. 273 aluminum sulfate + calcium hydroxide aluminum hydroxide + calcium sulfate Al 2(SO 4 )3 + 3 Ca(OH)2 2 Al(OH)3 + 3 Ca. SO 4 Al = 22 1 SO 4 =3 SO 4 =13 1 Ca = 13 1 OH = 6 2 OH = 66 3 Al 2(SO 4)3(aq) + 3 Ca(OH)2(aq) 2 Al(OH)3(s) + 3 Ca. SO 4(s) 26

Practice Problems 1. Write word, formula, and balanced chemical equations for each of the following reactions: a. Magnesium and hydrochloric acid react to produc magnesium chloride and hydrogen. b. Aqueous nitric acid reacts with solid magnesium hydroxide to produce aqueous magnesium nitrate and water 27

Practice Problems 2. Solid calcium metal reacts with water to form aqueous calcium hydroxide and hydrogen gas. Write a balanced chemical equation for this reaction. 28

Practice Problems 1. Write balanced chemical equations for each of the following reactions: a. Solid sodium combines with chlorine gas to produce solid sodium chloride. b. When solid copper reacts with aqueous silver nitrate, the products are aqueous copper (II) nitrate and solid silver c. In a blast furnace, the reaction between solid iron (III) oxide and carbon monoxide gas produces solid iron and carbon dioxide gas. 29

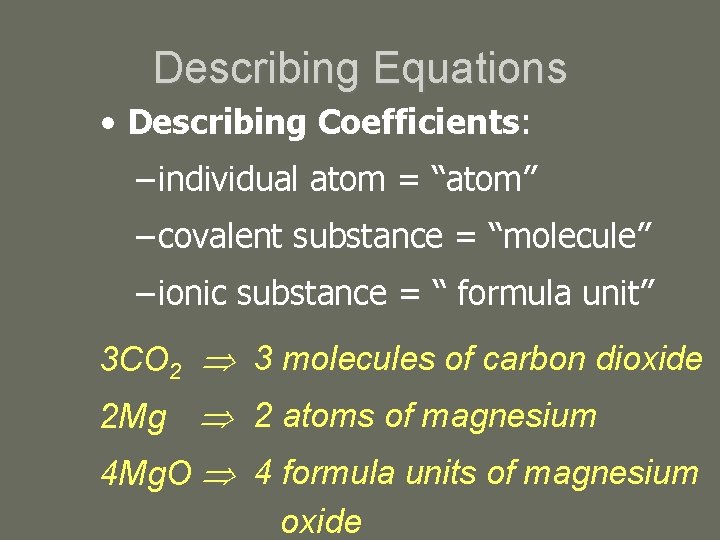
Describing Equations • Describing Coefficients: – individual atom = “atom” – covalent substance = “molecule” – ionic substance = “ formula unit” 3 CO 2 3 molecules of carbon dioxide 2 Mg 2 atoms of magnesium 4 Mg. O 4 formula units of magnesium oxide

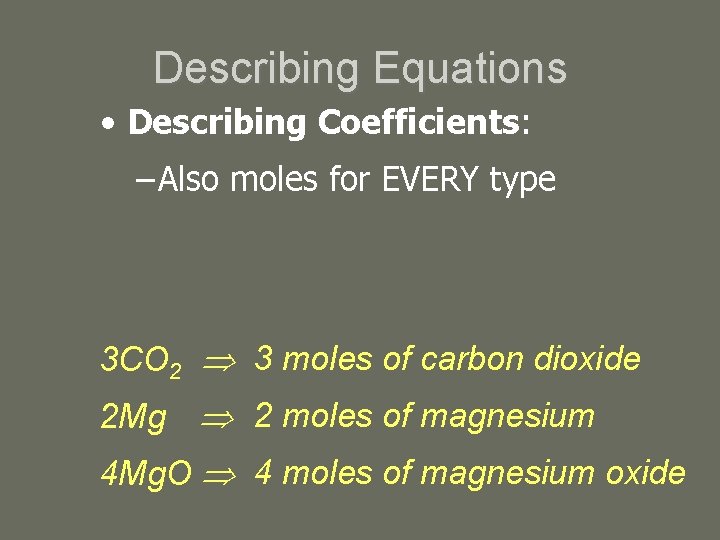
Describing Equations • Describing Coefficients: – Also moles for EVERY type 3 CO 2 3 moles of carbon dioxide 2 Mg 2 moles of magnesium 4 Mg. O 4 moles of magnesium oxide

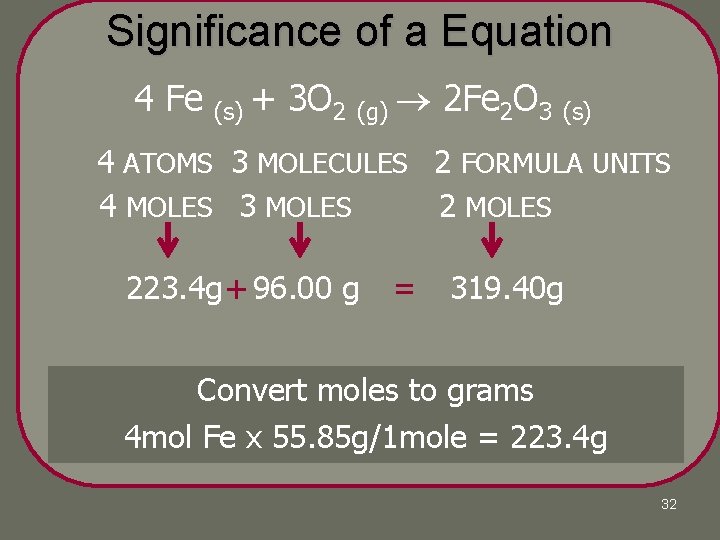
Significance of a Equation 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) 4 ATOMS 3 MOLECULES 2 FORMULA UNITS 4 MOLES 3 MOLES 223. 4 g + 96. 00 g = 319. 40 g Convert moles=to(or grams Coefficients = molecules moles formula units 4 mol x 55. 85 g/1 mole = 223. 4 g for Fe ionic or atoms for elements) 32

Types of Chemical Rx. N’s Section 2

Types of Rx. N’s • • • There are millions of reactions. Can’t remember them all Fall into several categories. We will learn 5 types. Will be able to predict the products. For some we will be able to predict whether they will happen at all. • Will recognize them by the reactants

Types of Rx. N’s • The classification scheme described in this section provides an introduction to five basic types of reactions: • Synthesis (Combination) • Decomposition • Single-displacement (replacement) • Double-displacement (replacement) • Combustion reactions

Synthesis Reactions • In a synthesis reaction, also known as a composition reaction or combination reaction, two or more substances combine to form a new compound. • This type of reaction is represented by the following general equation. A+X AX • A and X can be elements or compounds. • AX is a compound

Synthesis Reactions • 2 elements, or compounds combine to make one compound. • Ca +O 2 Ca. O • SO 3 + H 2 O H 2 SO 4 • We can predict the products if they are two elements. • Mg + N 2

Reactions of Elements with Oxygen • One simple type of synthesis reaction is the combination of an element with oxygen to produce an oxide of the element. • Almost all metals react with oxygen to form oxides. • example: 2 Mg(s) + O 2(g) 2 Mg. O(s) • Group 2 elements react in a similar manner, forming oxides with the formula MO, where M represents the metal.

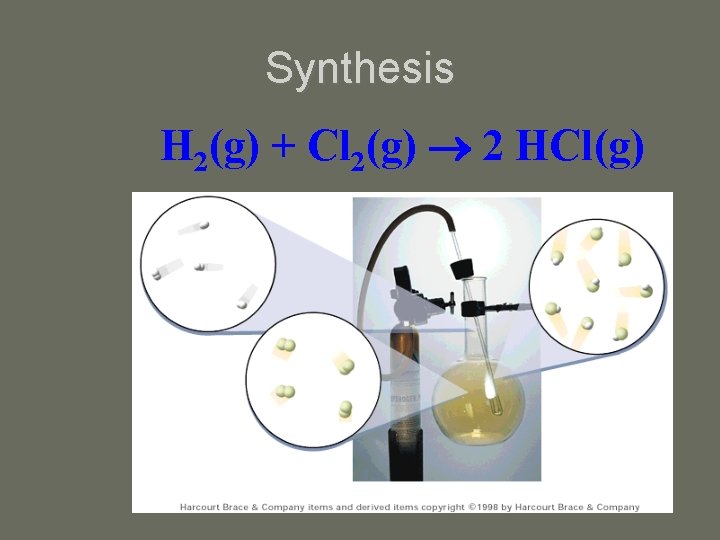
Synthesis H 2(g) + Cl 2(g) 2 HCl(g)

Your Turn III • • Ca + Cl 2 Fe + O 2 iron (II) oxide Al + O 2 Remember that the first step is to write the formula • Then balance

Decomposition Reactions • In a decomposition reaction, a single compound undergoes a reaction that produces two or more simpler substances. s or compounds. • Decomposition reactions are the opposite of synthesis reactions. • They are represented by the following general equation. AX A+X • AX is a compound. • A and X can be elements or compounds.

Decomposition of Binary Compounds • The decomposition of a substance by an electric current is called electrolysis. • example: • Oxides of the less-active metals, which are located in the lower center of the periodic table, decompose into their elements when heated. • example:

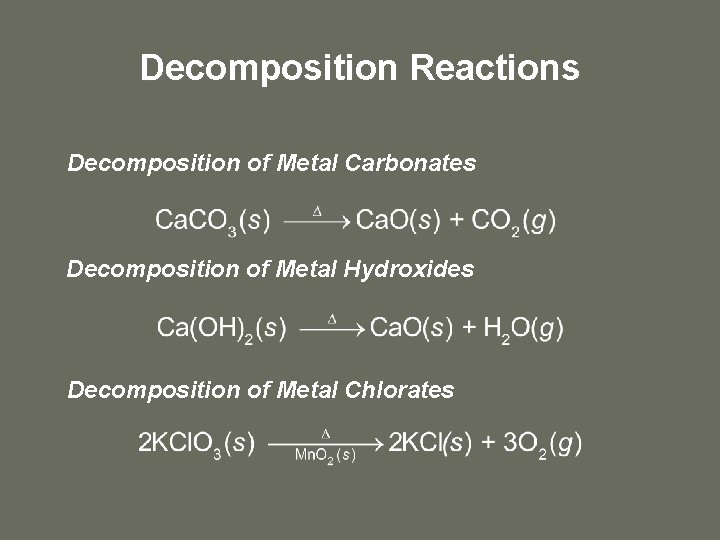
Decomposition Reactions Decomposition of Metal Carbonates Decomposition of Metal Hydroxides Decomposition of Metal Chlorates

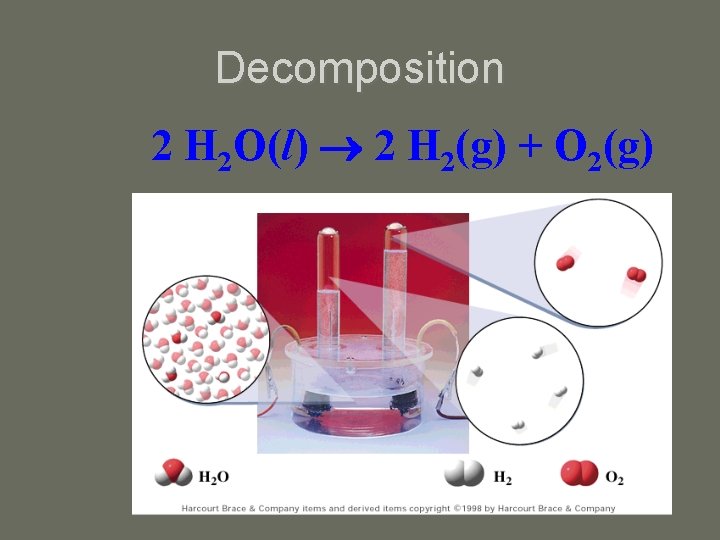
Decomposition 2 H 2 O(l) 2 H 2(g) + O 2(g)

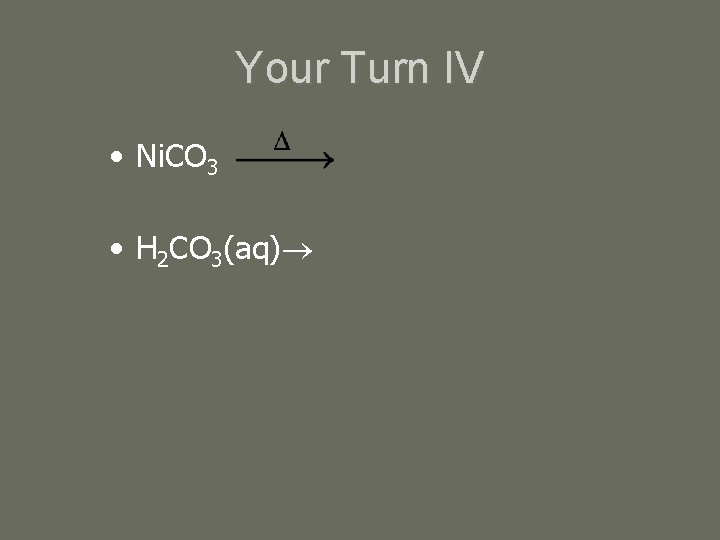
Your Turn IV • Ni. CO 3 • H 2 CO 3(aq)

Single-Replacement Reactions • In a single-replacement reaction, also known as a displacement reaction, one element replaces a similar element in a compound. • Many single-replacement reactions take place in aqueous solution. • Single-replacement reactions can be represented by the following general equations. A + BX AX + B or Y + BX BY + X • A, B, X, and Y are elements. AX, BX, and BY are compounds.

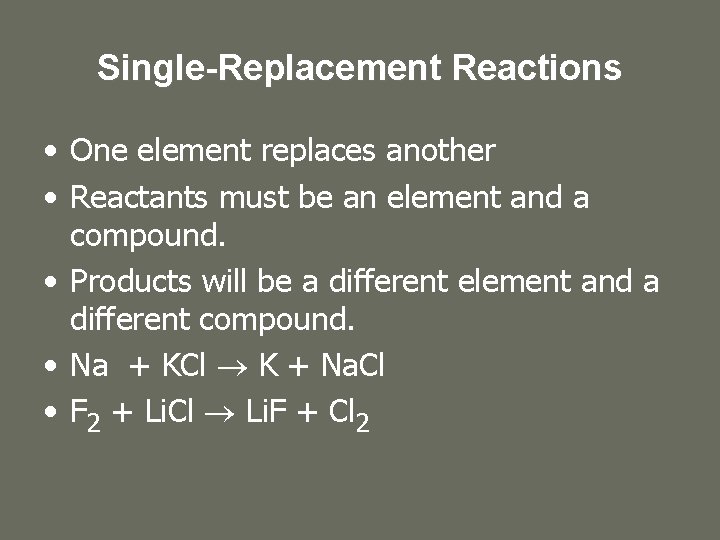
Single-Replacement Reactions • One element replaces another • Reactants must be an element and a compound. • Products will be a different element and a different compound. • Na + KCl K + Na. Cl • F 2 + Li. Cl Li. F + Cl 2

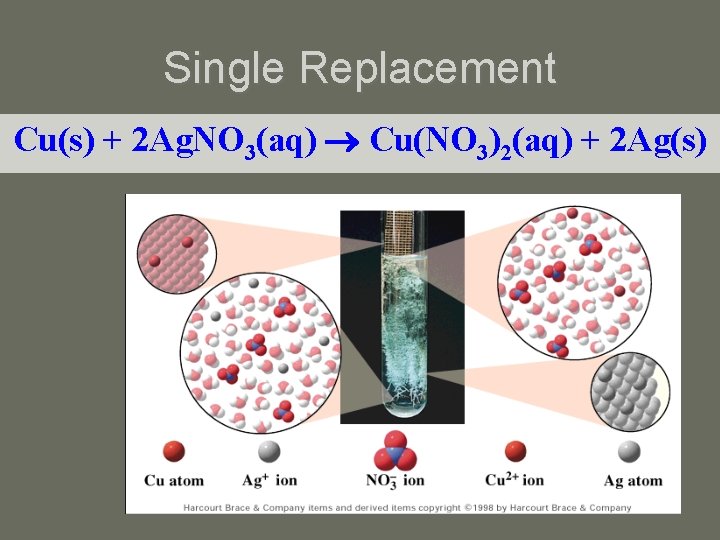
Single Replacement Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3)2(aq) + 2 Ag(s)

Single-Replacement Reactions • • • Metals replace metals (and hydrogen) K + Al. N Zn + HCl Think of water as HOH Metals replace one of the H, combine with hydroxide. • Na + HOH

Activity Series • • • We can tell whether a reaction will happen Some are more active than other More active replaces less active There is a list on page 286 Higher on the list replaces lower. If the element by itself is higher, it happens, if lower it doesn’t

Activity Series • H can be replaced in acids by everything higher • Only the first 6 (Li - Na) react with water. • Fe + Cu. SO 4 • Pb + KCl • Al + HCl

Activity Series • What does it mean that Au And Ag are on the bottom of the list? • Nonmetals can replace other nonmetals • Limited to F 2 , Cl 2 , Br 2 , I 2 • The order of activity is that on the table. • Higher replaces lower. • F 2 + HCl • Br 2 + KCl

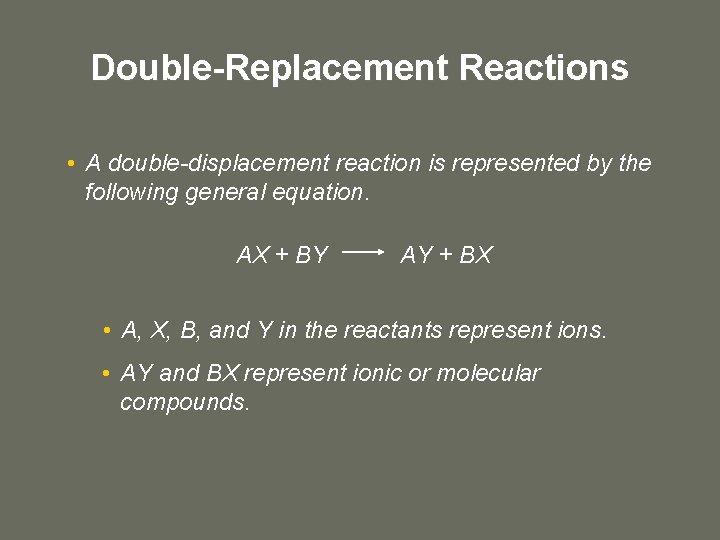
Double-Replacement Reactions • In double-replacement reactions, the ions of two compounds exchange places in an aqueous solution to form two new compounds. • One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of the solution, or a molecular compound, usually water. • The other compound is often soluble and remains dissolved in solution.

Double-Replacement Reactions • A double-displacement reaction is represented by the following general equation. AX + BY AY + BX • A, X, B, and Y in the reactants represent ions. • AY and BX represent ionic or molecular compounds.

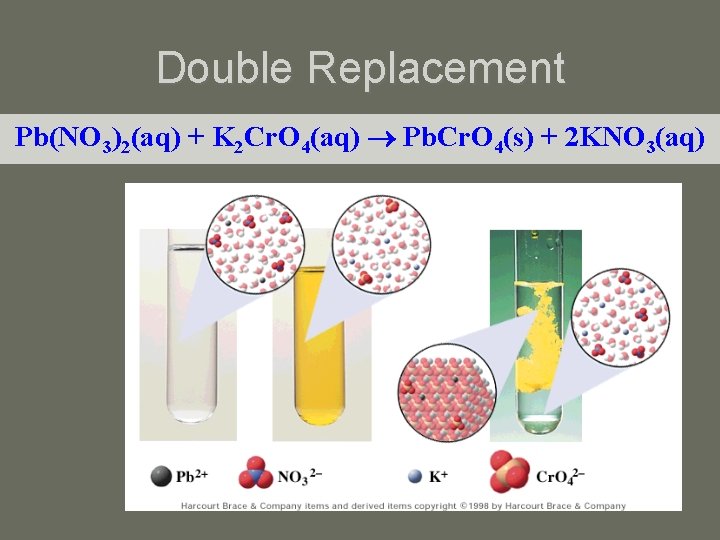
Double Replacement Pb(NO 3)2(aq) + K 2 Cr. O 4(aq) Pb. Cr. O 4(s) + 2 KNO 3(aq)

Double-Replacement Reactions • The formation of a precipitate occurs when the cations of one reactant combine with the anions of another reactant to form an insoluble or slightly soluble compound. • example: 2 KI(aq) + Pb(NO 3)2(aq) Pb. I 2(s) + 2 KNO 3(aq) • The precipitate forms as a result of the very strong attractive forces between the Pb 2+ cations and the I anions.

Double-Replacement Reactions • • Two things replace each other. Reactants must be two ionic compounds or acids. Usually in aqueous solution Na. OH + Fe. Cl 3 The positive ions change place. Na. OH + Fe. Cl 3 Fe+3 OH- + Na+1 Cl-1 Na. OH + Fe. Cl 3 Fe(OH)3 + Na. Cl

Double-Replacement Reactions • Will only happen if one of the products – doesn’t dissolve in water and forms a solid – or is a gas that bubbles out. – or is a covalent compound usually water.

Your Turn V • • • assume all of the reactions take place. Ca. Cl 2 + Na. OH Cu. Cl 2 + K 2 S KOH + Fe(NO 3)3 (NH 4)2 SO 4 + Ba. F 2

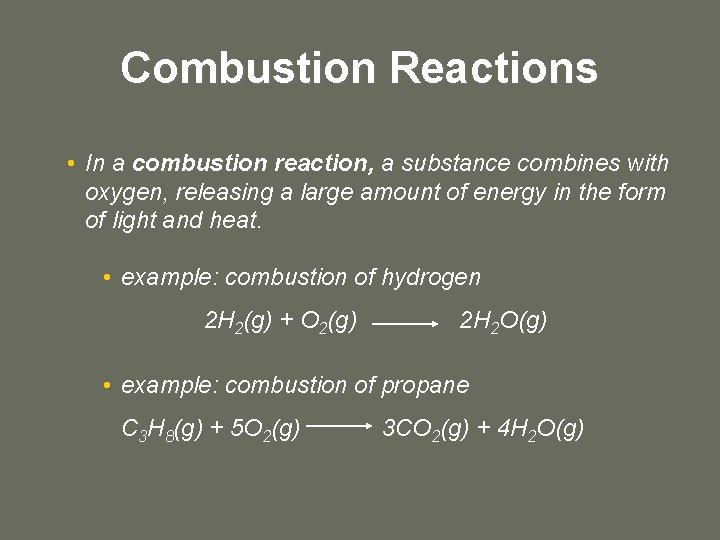
Combustion Reactions • In a combustion reaction, a substance combines with oxygen, releasing a large amount of energy in the form of light and heat. • example: combustion of hydrogen 2 H 2(g) + O 2(g) 2 H 2 O(g) • example: combustion of propane C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g)

Combustion Reactions • Combustion • A compound composed of only C H and maybe O is reacted with oxygen • If the combustion is complete, the products will be CO 2 and H 2 O. • If the combustion is incomplete, the products will be CO and H 2 O.

Your Turn VI • C 4 H 10 + O 2 (complete) • C 4 H 10 + O 2 (incomplete) • C 6 H 12 O 6 + O 2 (complete) • C 8 H 8 +O 2 (incomplete)

Your Turn VI Part II Distinguish between complete an incomplete combustion. Write a balanced equation for the complete combustion of each of these compounds. a) acetic acid, HC 2 H 3 O 2 c) glycerol, C 3 H 8 O 3 b) decane, C 10 H 22 d) sucrose, C 12 H 22 O 11 Write a balanced equation for the incomplete combustion of each of these compounds. a) glycerol, C 3 H 8 O 3 c) acetic acid, HC 2 H 3 O 2 b) glucose, C 6 H 12 O 6 d) acetylene, C 2 H 2

Your Turn VII • Determine the type of reaction and the products and balance. • H 2 + O 2 • H 2 O • Zn + H 2 SO 4 • Hg. O • KBr +Cl 2 • Ag. NO 3 + Na. Cl • Mg(OH)2 + H 2 SO 3

Let’s Review • Write out the balanced equation for Copper reacts with chlorine to form copper (II) chloride. • Write out the balanced equation for Zinc reacts with Hydrochloric acid to form Zinc chloride and hydrogen. • Write out the balanced equation for Calcium carbonate reacts with Zinc nitride to form Calcium nitride and Zinc carbonate.

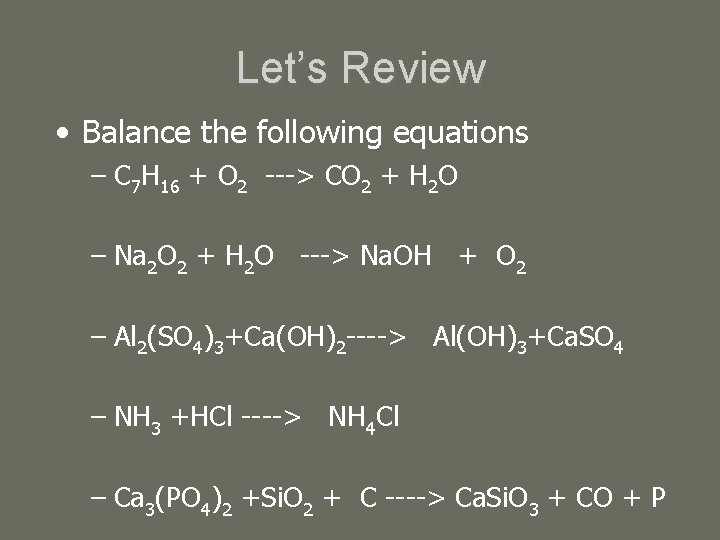
Let’s Review • Balance the following equations – C 7 H 16 + O 2 ---> CO 2 + H 2 O – Na 2 O 2 + H 2 O ---> Na. OH + O 2 – Al 2(SO 4)3+Ca(OH)2 ----> Al(OH)3+Ca. SO 4 – NH 3 +HCl ----> NH 4 Cl – Ca 3(PO 4)2 +Si. O 2 + C ----> Ca. Si. O 3 + CO + P

Let’s Review • What type of reaction are the following – – – Rb + S 8 ---> Rb 2 S NH 3 + O 2 ---> N 2 + H 2 O C + SO 2 ---> CS 2 + CO C 3 H 11 + O 2 ---> CO 2 + H 2 O C 5 H 12 +O 2 ---> CO + H 2 O Fe. Cl 3 + NH 4 OH --->Fe(OH)3 + NH 4 Cl

Let’s Review • Balance them – – – Rb + S 8 ---> Rb 2 S NH 3 + O 2 ---> N 2 + H 2 O C + SO 2 ---> CS 2 + CO C 3 H 11 + O 2 ---> CO 2 + H 2 O C 5 H 12 +O 2 ---> CO + H 2 O Fe. Cl 3 + NH 4 OH --->Fe(OH)3 + NH 4 Cl

Let’s Review • Predict the products and balance – Sodium phosphate reacts with Lithium metal. – Butane (C 4 H 10) is completely combusted. – Sodium Chloride reacts with Silver Hydroxide – Aluminum Bromate reacts with Copper – Potassium reacts with Sulfur – Octane (C 8 H 18) is incompletely combusted

Let’s Review • Predict the products and balance – – – – H 2 + O 2 Al 2 Te 3 Zn + H 2 SO 4 Hg. O KBr +Cl 2 Ag. NO 3 + Na. Cl Mg(OH)2 + H 2 SO 3

Let’s Review • Using the table on Pg 286. Predict if these reactions will occur – – – – Li + Ba. S Fe + KCl Au + Hg. S Mn + Cu. S Ba + Li. S Na + KCl HCl + Pb Pb. Cl 2 + H 2
 Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Are kc and kp equal
Are kc and kp equal Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Synthesis reaction
Synthesis reaction Types of reactions
Types of reactions Unit 5 chemical reactions
Unit 5 chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chapter 6 chemistry in biology
Chapter 6 chemistry in biology Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Toxic reactions chemical equations worksheet answers
Toxic reactions chemical equations worksheet answers Toxic reactions chemical equations
Toxic reactions chemical equations Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key Chapter 9 chemical reactions study guide
Chapter 9 chemical reactions study guide Translate word equations to chemical equations
Translate word equations to chemical equations Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. 5 type of reactions
5 type of reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions What are redox reactions examples
What are redox reactions examples Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Trinitrogen monosulfide formula
Trinitrogen monosulfide formula Chemical reactions reactants and products
Chemical reactions reactants and products Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Reactions of copper and percent yield
Reactions of copper and percent yield What is released or absorbed whenever chemical
What is released or absorbed whenever chemical Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Building vocabulary: chemical bonds and reactions
Building vocabulary: chemical bonds and reactions Modern chemistry chapter 9
Modern chemistry chapter 9 Modern chemistry chapter 15
Modern chemistry chapter 15 Chapter 14 acids and bases
Chapter 14 acids and bases Chapter 13 review ions in aqueous solutions
Chapter 13 review ions in aqueous solutions Modern chemistry chapter 12 test
Modern chemistry chapter 12 test Modern chemistry chapter 4
Modern chemistry chapter 4 Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities 4 types of chemical reactions
4 types of chemical reactions 5 types of reactions in chemistry
5 types of reactions in chemistry Type of reactions chemistry
Type of reactions chemistry Types of reactions chemistry
Types of reactions chemistry Slidetodoc.com
Slidetodoc.com Stoichiometry mole island diagram
Stoichiometry mole island diagram Balancing redox reactions
Balancing redox reactions Identify types of reactions
Identify types of reactions Tyoes of chemical reactions
Tyoes of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Principles of immuno chemical reactions
Principles of immuno chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions More predicting products of chemical reactions
More predicting products of chemical reactions Activity series of metals
Activity series of metals Unit 11 chemical reactions
Unit 11 chemical reactions Four types of chemical reactions
Four types of chemical reactions Www.biology-roots.com
Www.biology-roots.com Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Chemical reactions in everyday life
Chemical reactions in everyday life 5 general types of chemical reactions
5 general types of chemical reactions 5 general types of chemical reactions
5 general types of chemical reactions Solubility rules
Solubility rules Rate constant and equilibrium constant
Rate constant and equilibrium constant What are the 4 types of chemical reactions
What are the 4 types of chemical reactions