Modern Biology Chapter 2 Chemistry of Life Composition

Modern Biology Chapter 2 - Chemistry of Life
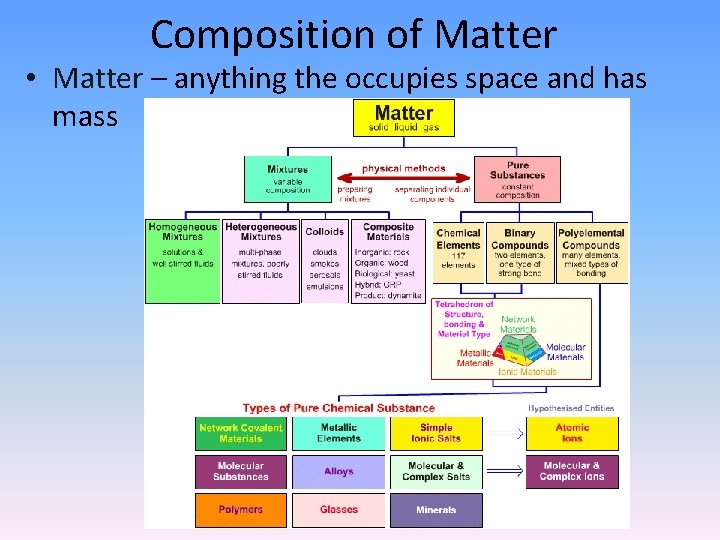
Composition of Matter • Matter – anything the occupies space and has mass

Composition of Matter • Mass – quantity of matter an object has – Weight – gravity acting on mass
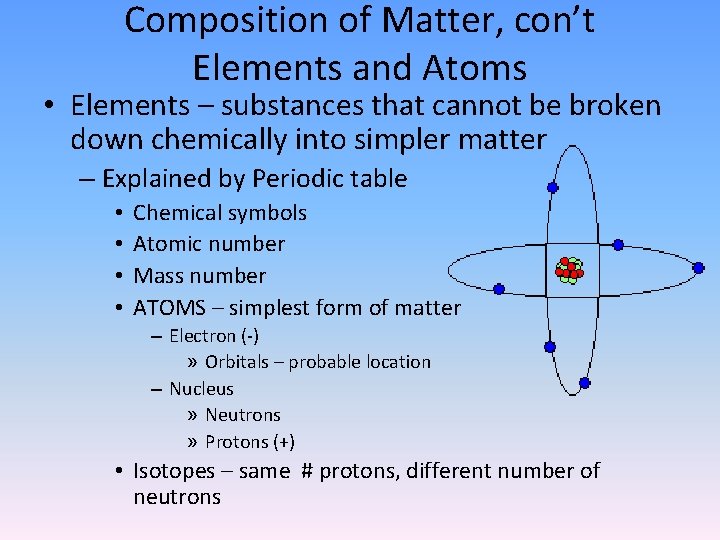
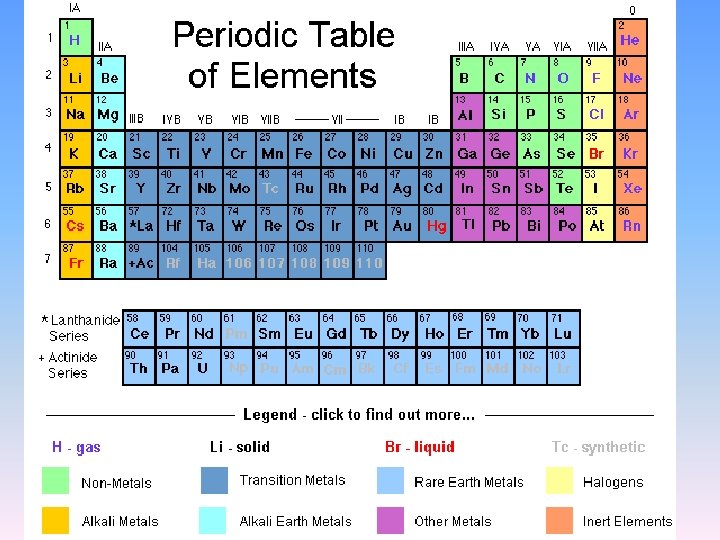
Composition of Matter, con’t Elements and Atoms • Elements – substances that cannot be broken down chemically into simpler matter – Explained by Periodic table • • Chemical symbols Atomic number Mass number ATOMS – simplest form of matter – Electron (-) » Orbitals – probable location – Nucleus » Neutrons » Protons (+) • Isotopes – same # protons, different number of neutrons
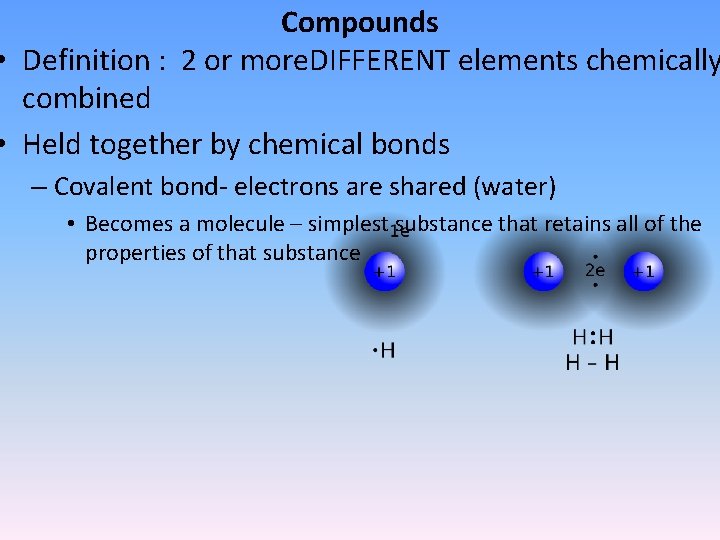
Compounds • Definition : 2 or more. DIFFERENT elements chemically combined • Held together by chemical bonds – Covalent bond- electrons are shared (water) • Becomes a molecule – simplest substance that retains all of the properties of that substance
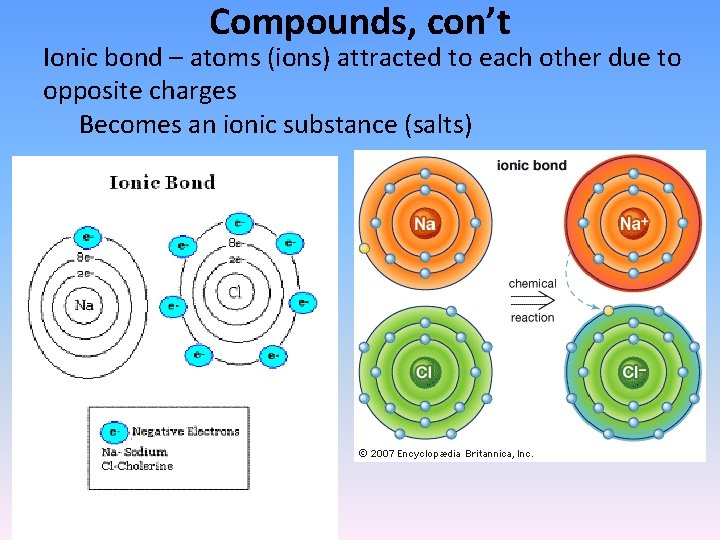
Compounds, con’t Ionic bond – atoms (ions) attracted to each other due to opposite charges Becomes an ionic substance (salts)
Energy • Energy – the ability to do work – Types found in living organisms: • • Chemical energy Thermal energy Electrical energy Mechanical energy
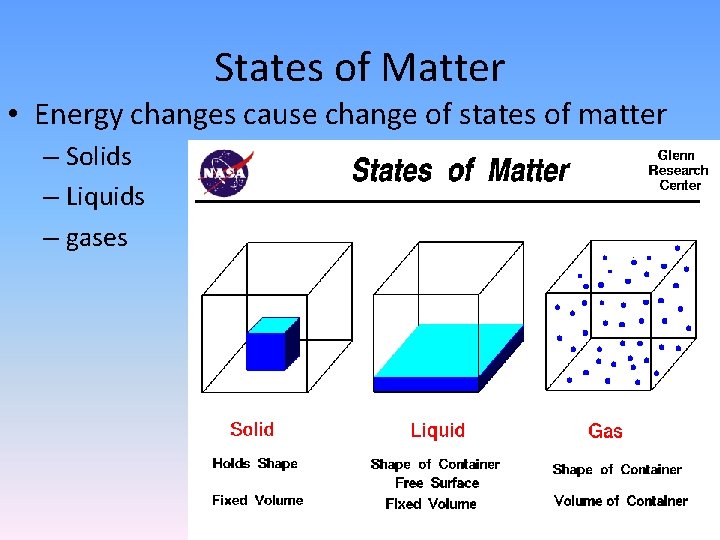
States of Matter • Energy changes cause change of states of matter – Solids – Liquids – gases
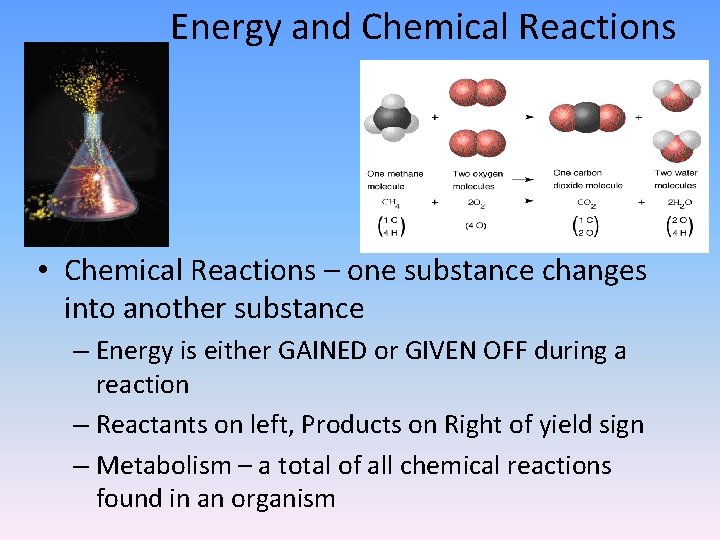
Energy and Chemical Reactions • Chemical Reactions – one substance changes into another substance – Energy is either GAINED or GIVEN OFF during a reaction – Reactants on left, Products on Right of yield sign – Metabolism – a total of all chemical reactions found in an organism
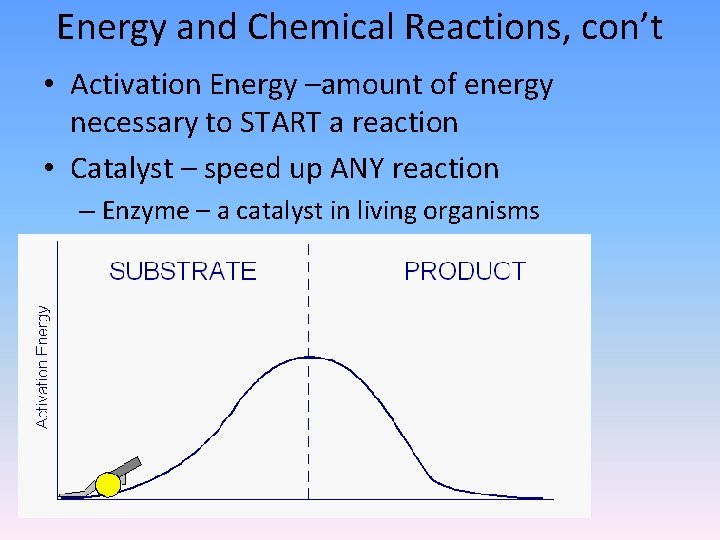
Energy and Chemical Reactions, con’t • Activation Energy –amount of energy necessary to START a reaction • Catalyst – speed up ANY reaction – Enzyme – a catalyst in living organisms
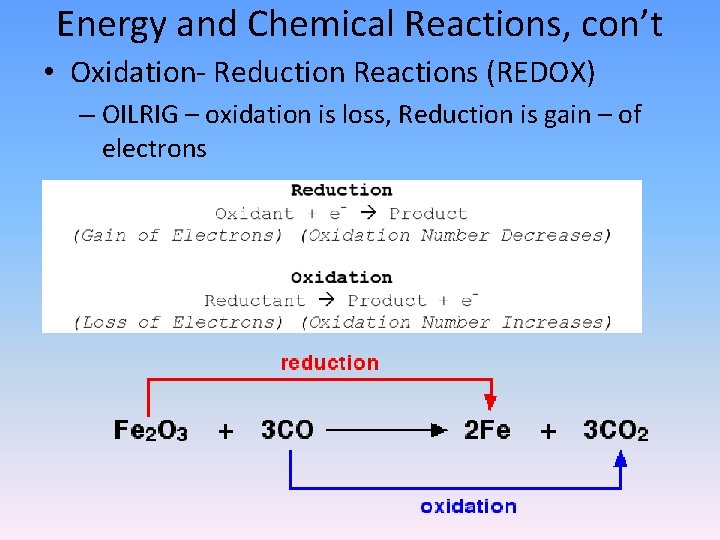
Energy and Chemical Reactions, con’t • Oxidation- Reduction Reactions (REDOX) – OILRIG – oxidation is loss, Reduction is gain – of electrons
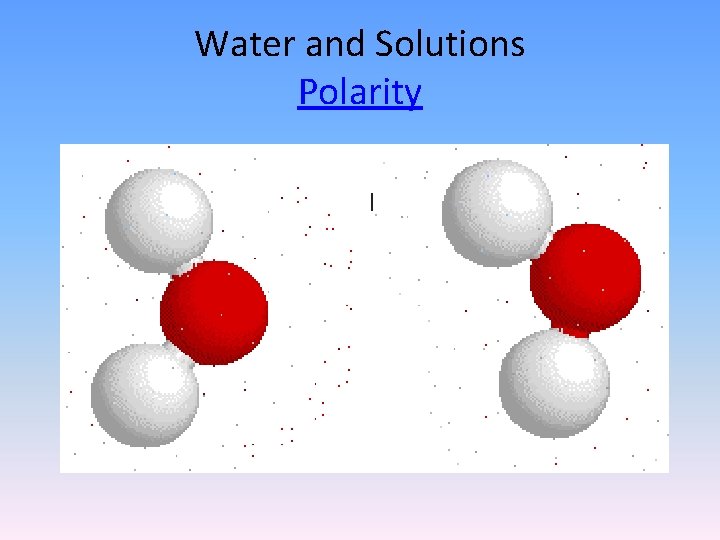
Water and Solutions Polarity
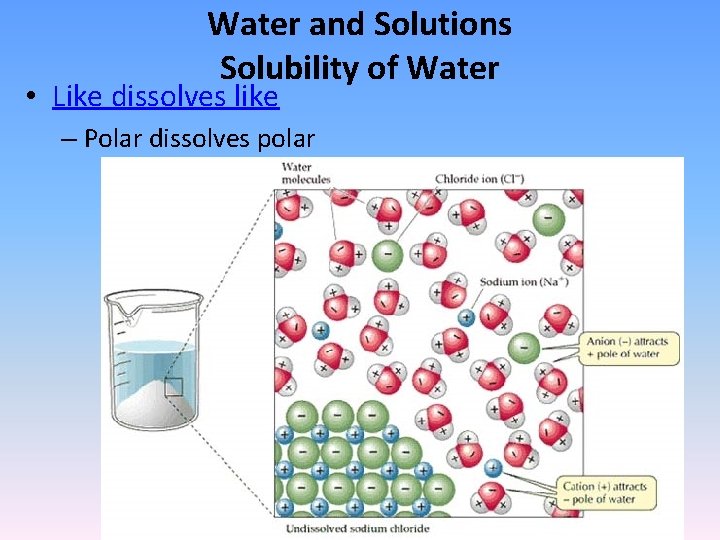
Water and Solutions Solubility of Water • Like dissolves like – Polar dissolves polar
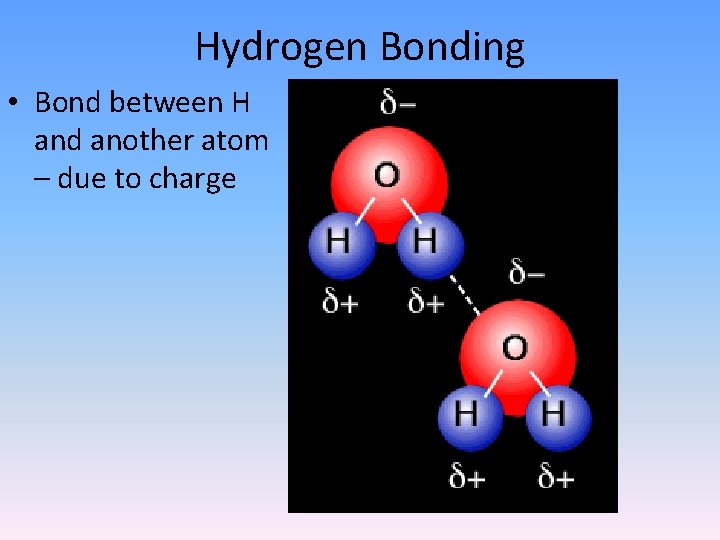
Hydrogen Bonding • Bond between H and another atom – due to charge

Cohesion and Adhesion • Cohesion due to H bond – Holds molecules of a SINGLE substance together • Adhesion- attractive force between two different substances – Capillary action (fluid rises) (meniscus) cohesion Adhesion

Solutions • Solution – solutes are evenly distributed – Solutes – dissolve in a solvent – Universal solvent – water – Concentration – how much solvent is dissolved in the solute – Saturated solution – one that can not hold ANY more solvent at room temperature – Aqueous solutions – when water is the solvent • Virtually ALL living organisms

Acids and Bases • Acid – extra H attaches to water making hydronium ions (H 3 O) in water. ( a H – ion for short) Nitric Acid - HNO 3 Nitrous Acid - HNO 2 Hypochlorous Acid - HCl. O Chlorous Acid - HCl. O 2 Chloric Acid - HCl. O 3 Perchloric Acid - HCl. O 4 Sulfuric Acid - H 2 SO 4 Sulfurous Acid - H 2 SO 3 Phosphoric Acid - H 3 PO 4 Phosphorous Acid - H 3 PO 3 Carbonic Acid - H 2 CO 3 Acetic Acid - HC 2 H 3 O 2 Oxalic Acid - H 2 C 2 O 4 Boric Acid - H 3 BO 3 Silicic Acid - H 2 Si. O 3 Sodium Hydroxide - Na. OH Potassium Hydroxide - KOH Ammonium Hydroxide - NH 4 OH Calcium Hydroxide - Ca(OH)2 Magnesium Hydroxide - Mg(OH)2 Barium Hydroxide - Ba(OH)2 Aluminum Hydroxide - Al(OH)3 Ferrous Hydroxide or Iron (II) Hydroxide Fe(OH)2 Ferric Hydroxide or Iron (III) Hydroxide Fe(OH)3 Zinc Hydroxide - Zn(OH)2 Lithium Hydroxide - Li. OH • Bases – the presence of OH (hydroxide ion) in solution
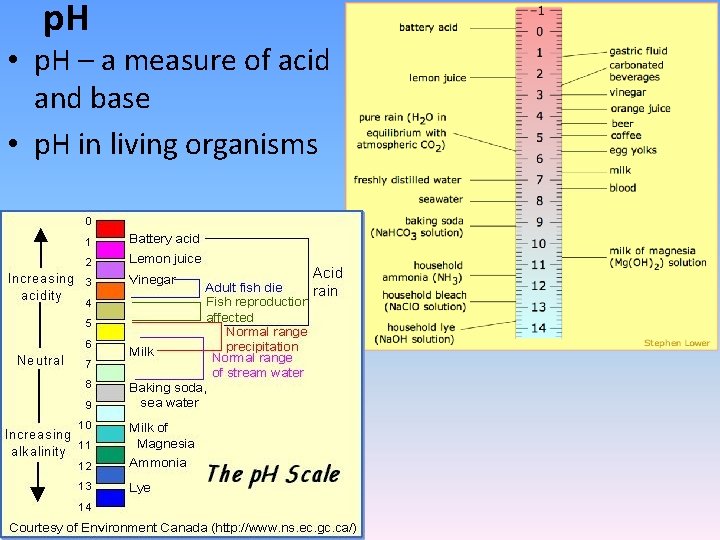
p. H • p. H – a measure of acid and base • p. H in living organisms
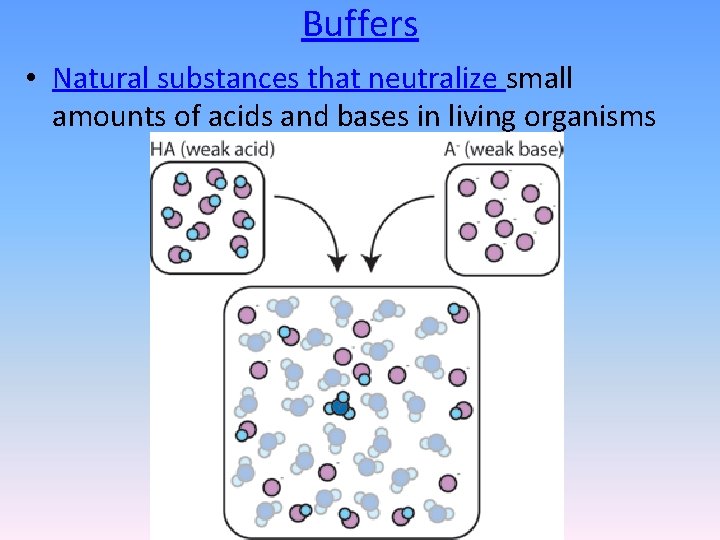
Buffers • Natural substances that neutralize small amounts of acids and bases in living organisms
- Slides: 20